In China, veterinary drugs require registration with the Chinese Ministry of Agriculture and Rural Affairs (MARA) before they can be produced, imported, sold, or used in China. Veterinary drugs are defined as substances (including pharmaceutical feed additives) used to prevent, treat, diagnose animal diseases or purposefully regulate animal physiological functions. They typically include the following product categories.
- Chemical drugs
- Biological products
- Chinese medicines
- Diagnostic reagents
- Disinfectants
Authorities
- The Ministry of Agriculture and Rural Affairs (MARA) in China
- Institute of Veterinary Drug Control (IVDC) in China
Main Veterinary Drug Regulations in China
- Regulations on the Administration of Veterinary Drugs (State Council Decree 404, 2004)
- Measures for the Registration of Veterinary Drugs (MARA Order 44, 2004)
- Data Requirements for the Registration of Veterinary Drugs (MARA Order 442, 2004)
- Measures of the Development of New Veterinary Drugs
Data Requirements for Veterinary Drug Registration in China
Depending on product type and whether a product belongs to new veterinary drugs, data requirements vary significantly. A new veterinary drug is defined as a veterinary drug (including active ingredient) that has NOT been sold in China before.
Required data usually includes product summaries, pharmacological data, toxicological data, efficacy data (for disinfectants), residue data and/or clinical trial data. Clinical trial data is not required for active ingredients. Clinical trials must be conducted in accordance with GCP.
Other required documents include approval documents issued by other authorities, product standards and sample labels.
For imported veterinary drugs, it is recommended to use a Chinese agent to arrange all studies, prepare registration dossier and communicate with MARA.
Veterinary Drug Registration Process in China
The picture below describes the whole veterinary drug registration review and approval process in China. 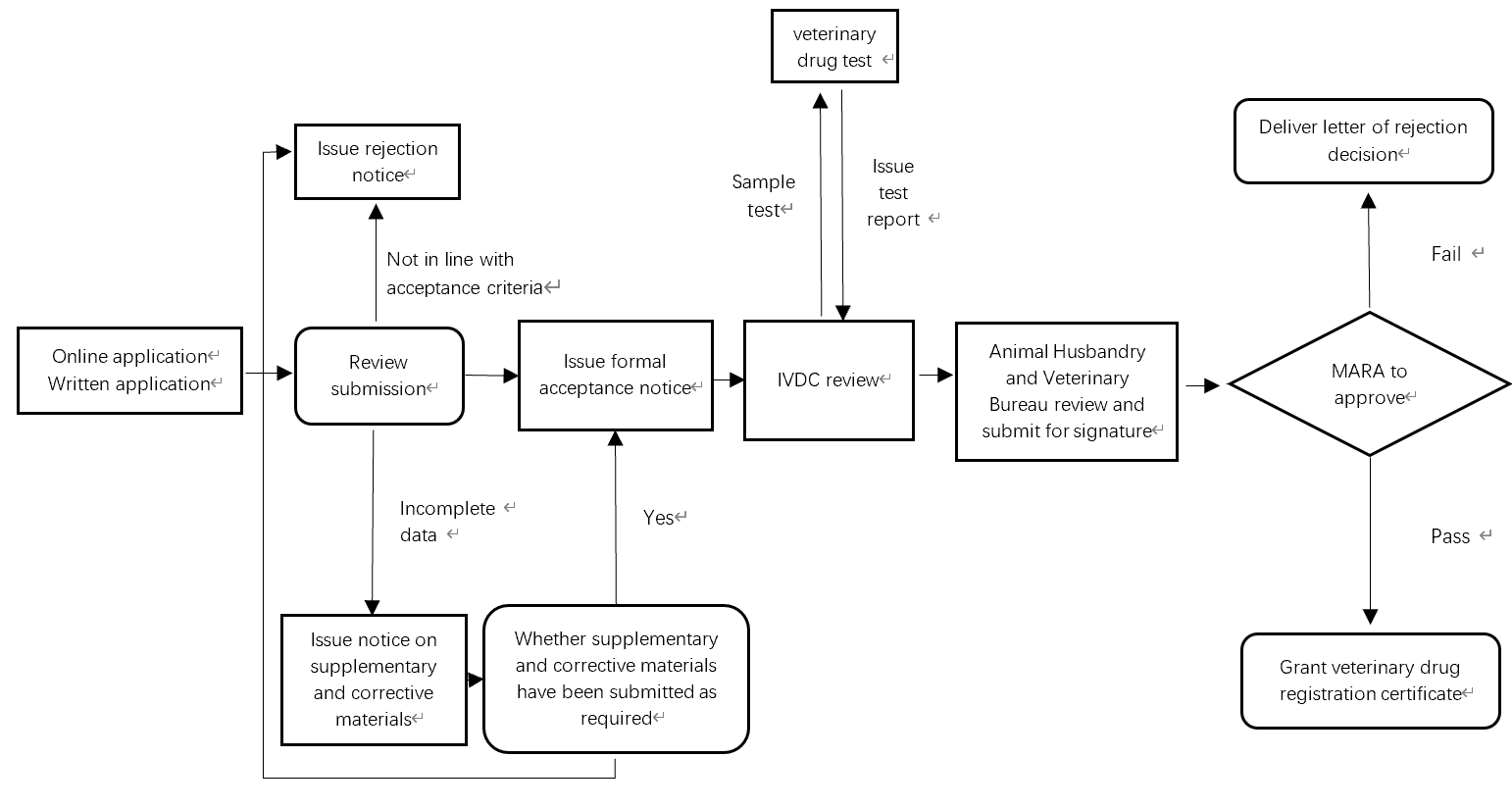
Depending on product type, it takes 1.5-4 years to register a veterinary drug in China.
Our Services
- Data Gap Analysis/Registration Feasibility Evaluation;
- Coordination of Clinical Trials in China;
- Registration of Veterinary Drugs in China;
- Regulatory Advice and Training;