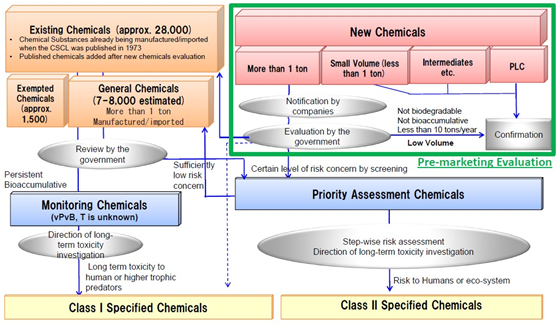
The Amended Japan CSCL issued by Ministry of Economy, Trade and Industry; Ministry of Health, Labour and Welfare, Ministry of the Environment enterd into force in two steps on 1 April 2009 and 1 April 2011.
For new chemicals, manufacturers or importers (M/I’s) in Japan shall comply with the requirement under Japan CSCL. The main obligation of new chemical under CSCL is described as following:
- Notification of new chemical;
- Annual report
For other chemicals including General Chemicals, Priority Assessment Chemicals (PAC’s) and Monitoring Chemicals, annul report is always required. For more detailed information, please see the table of Requirements for chemicals other than new chemicals.
Evaluation/Assessment Flow of CSCL
Whether Pre-marketing New Chemical Notification is Necessary
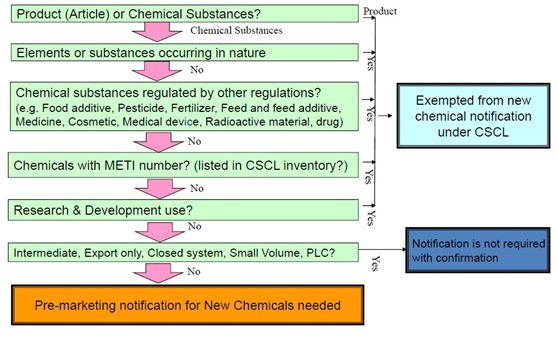
Required Actions for New Substance Chemicals under CSCL
Procedure type | Notification type | Requirements |
Notification | Standard notification | Different testing data depends on biodegradability of the substance. |
| Low volume confirmation (New substances ≤ 10t/y) | The substance is confirmed as non-biodegradable, non-bio-accumulative and the total volume of the substance manufactured/imported in Japan is ≤ 10 t/y. |
| Small amount confirmation (New substances ≤ 1t/y) | No testing data is required, the total volume of the manufactured/imported substance in Japan is ≤ 1t/y. |
| Intermediates | M/I’s may apply for confirmation instead of the full notification. |
| Export only | |
| Polymer of low concern |
Requirements for Chemicals other than New Chemicals
Chemical type | Requirements | |
Existing chemicals | General chemicals | Annual reporting of the volume of M/I uses. |
Exempt chemicals | No requirements | |
Priority assessment chemicals(PACs) | Annual reporting of the volume of M/I uses if the volume is > 1t/y. | |
Monitoring chemicals | Annual reporting of the quanity and the use is required after M/I, if the volume is > 1kg/y. | |
Class 1 specified chemicals | M/I is banned unless authorised. Articals/products which use a class 1 specified chemicals shall not be imported. | |
Class 2 specified chemicals | Notification of planned quantity of manufacture or import should be submitted a month before manufacture or import of the chemical. | |
How to Check the Status of a Chemical
J-Check: provides information regarding CSCL, such as the lists of CSCL, chemical safety information obtained in the existing chemicals survey programme and the Japan Challenge Programme in cooperation with eChemPortal by OECD.
http://www.safe.nite.go.jp/jcheck/top.action
NITE CHRIP: comprehensive information on risk assessments, laws and regulations of chemical substances that can be searched by entering a number or name, can also provide a list for each category.

