Background
Perfume and makeup products are two of the most widely imported cosmetic products to mainland China from the EU and US. They are considered cosmetic products in China because they meet the definition of cosmetic according to the overarching Cosmetic Supervision & Administration Regulation (CSAR), implemented in 2021 by the National Medical Products Administration (NMPA).
According to the regulation, cosmetics are defined as daily chemical industrial products applied to skin, hair, nails, lips, and other human surfaces by scrubbing, spraying, or other similar methods for the purpose of cleansing, protecting, beautifying, and modifying.
The management process for importing perfume and makeup products is determined by their classification in China. In general, these products are classified as ordinary cosmetics but certain claims such as SPF protection or use on children/infants may result in a special classification. Perfume and makeup products without special claims are considered ordinary cosmetics and are subject to filing management. Those with special claims require registration management.
Special | Ordinary | Other |
Hair Dye | Cosmetics that are not special cosmetics | Toothpaste |
Hair Perm | ||
Freckle-removing | ||
Sunscreens | Soap (without a special claim) | |
Anti-hair Loss | ||
Products with New Efficacy |
*Products with new efficacy refers to cosmetics with efficacies and application methods not listed in the Classification Rules & Catalog, as well as cosmetics targeted at children, pregnant women, and breastfeeding women.
An important consideration for overseas companies based in the EU and US is whether they have a legal entity based in mainland China or a domestic responsible person (DRP). This is a mandatory requirement of the regulation.
The DRP should meet the following conditions:
- Be a registered legal entity in mainland China
- Have a business license in the scope of cosmetics and relating to import/export
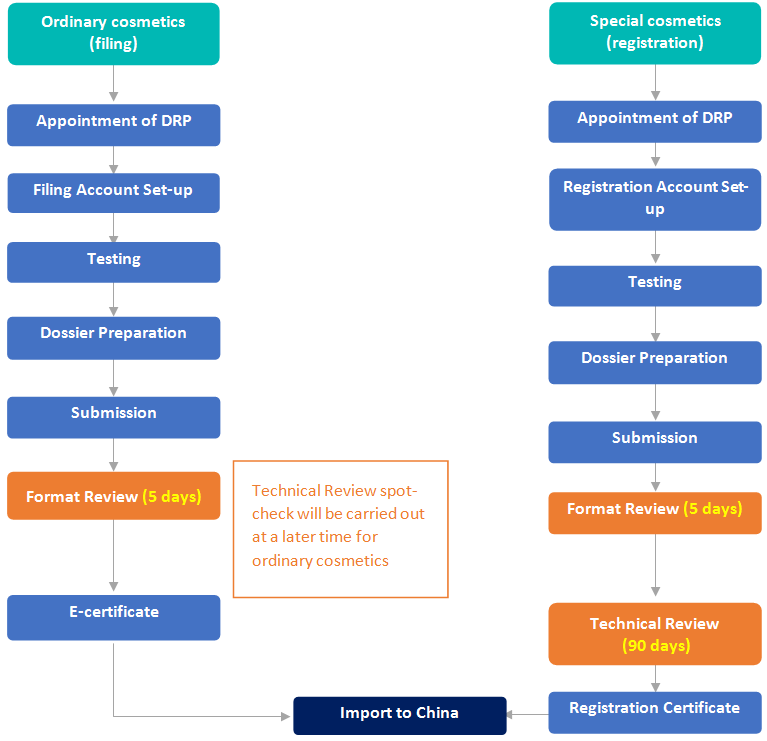
Filing/Registration of Perfume & Makeup Products in China
Animal Testing Exemption Requirements for Perfume & Makeup Products
For perfume and makeup products without special claims, i.e., Ordinary cosmetics in China, animal testing may be exempted under certain conditions:
- It is classified as ordinary cosmetic
- GMP/ISO certificate issued by the government of the country of origin
- Safety Assessment Report verifies the safety of the product
- There are no claims for use by infants and children
- Product formula only contains ingredients that have passed the three-year monitoring period and have been included in the Inventory of Existing Cosmetic Ingredients (IECIC 2021)
- Filer/Registrant is not be subject to supervision by the NMPA
Reporting the Safety Information of Cosmetic Ingredients to the NMPA
According to the CSAR regulation, the safety information of cosmetic ingredients used in the product formula of perfume and makeup should be reported to the NMPA before applicable deadlines:

There are two ways to report the information:
- Filer/registrant can complete Annex 14 for each cosmetic ingredient and submit with filing/registration dossier
- The supplier of the cosmetic ingredients responsible for the safety can generate a code on the NMPA safety information platform and share it with downstream filer/registrants
Practical Tips for Importing Perfume & Makeup Products to China
- Create an inventory list of perfume and makeup products manufactured/traded by your company.
Examples of terms used:
Perfumes | Makeup |
Perfume/Parfum | Skin makeup (foundation, concealer, blusher, highlighter, bronzer) |
Eau de Parfum (EDP) | Eye makeup (eyeshadow, eyeliner, mascara) |
Eau de Toilette | Lip makeup (lipstick, lip gloss, lip liner, lip balm) |
Eau de Cologne | Eyebrow makeup (eyebrow pencil, eyebrow gel) |
Eau Fraiche | Nail makeup (nail polish, nail glitter) |
Note: they should also meet the definition of cosmetic according to the CSAR regulation | |
- Determine the classification in China. In general, perfumes and makeup products are considered ordinary cosmetics but there are some exceptions if they have a special claim.
Examples of special claims for perfume and makeup products include foundation with SPF 30, nail glitter for kids, and SPF lip balm.
- Determine the management type based on the classification: filing management (ordinary) or registration management (special).
- Determine if you have a DRP in China or contact a third party (e.g., CIRS) for this service.
- Consider if waiving animal testing is a priority. Remember, this only applies to ordinary cosmetics which meet certain exemption criteria (see above).
- Check with your raw material suppliers if they have NMPA safety information codes or are willing to share the safety information with you to fill in Annex 14 for each raw material in the product formula.
Comparison of Perfume and Makeup Compliance in the EU, US, and China
EU | US | China | |
Authority | EU Commission | FDA | NMPA |
Regulation | Regulation (EC) N° 1223/2009 | The Federal Food, Drug and Cosmetic Act (FD&C Act) & Fair Packaging & Label Act (FPLA) | CSAR Regulation |
Classification | Cosmetic | Cosmetic | Ordinary Cosmetic |
Registrant | Legal Entity established in the EU (Responsible Person) | Legal Entity in the US | Legal Entity in mainland China (Domestic Responsible Person) |
Dossier Type | Product Information File (PIF) | N/A | Filing Dossier |
Safety Information | Cosmetic Product Safety Report (CPSR) prepared by a qualified Safety Assessor | Cosmetic Product Ingredient Statements | Safety Assessment Report prepared by qualified Safety Assessor; NMPA platform code or Annex 14 for raw materials |
Dossier Submission | Cosmetic Product Notification Portal (CPNP) | Voluntary Cosmetic Registration Program (VCRP) | NMPA Filing Account |
Animal Testing Requirement | Banned | Optional | Ordinary Cosmetic exemption only (must meet certain conditions; see above) |
Pre-market Authority Review Process | No official pre-market approval required before import. The RP should have a PIF always readily available for authority review check | No official pre-market approval required before import (unless colour additive) | Format Review (5 working days) before issuing e-cert for import to China. Technical Review spot-check at a later date |
Conclusion
The market for perfume and makeup products from the EU and US remains very popular in China. Therefore, companies based in these regions should be aware of the regulatory requirements before importing to China. Perfume and makeup products are classified as ordinary cosmetics unless they have a special function claim. Their management, be it filing, or registration is subject to their classification. The advantage of filing management includes waiving the animal testing requirement under certain conditions and reducing authority review time before compliantly introducing the products to the Chinese market. If there is a special claim, animal testing is mandatory and a full technical review by the NMPA is required before import and sale to China.
CIRS Services
- Domestic Responsible Person Service (CIRS Beijing)
- Finished Cosmetic Product Service (Filing & Registration)
- New Cosmetic Ingredient Registration
- Technical Assistance for Submission to the NMPA Safety Information Platform
- Testing Coordination including Efficacy Evaluation
- Regulatory Consultancy & Training

Julie Harrington, Senior Regulatory Consultant (CIRS EU)

Chris Ketchum, Senior Regulatory Consultant (CIRS US)
For further information, please contact us at service@cirs-group.com.
On October 25, Julie and Christopher will also be hosting a webinar that will explore the process of importing makeup and perfume products into the Chinese market. It will cover the journey from beginning to end, touching on some of the challenges as well as the practicalities such as labeling requirements and upcoming deadlines. They will provide some practical tips and insights from a European and American perspective.
You can find more information and register on our events page here.
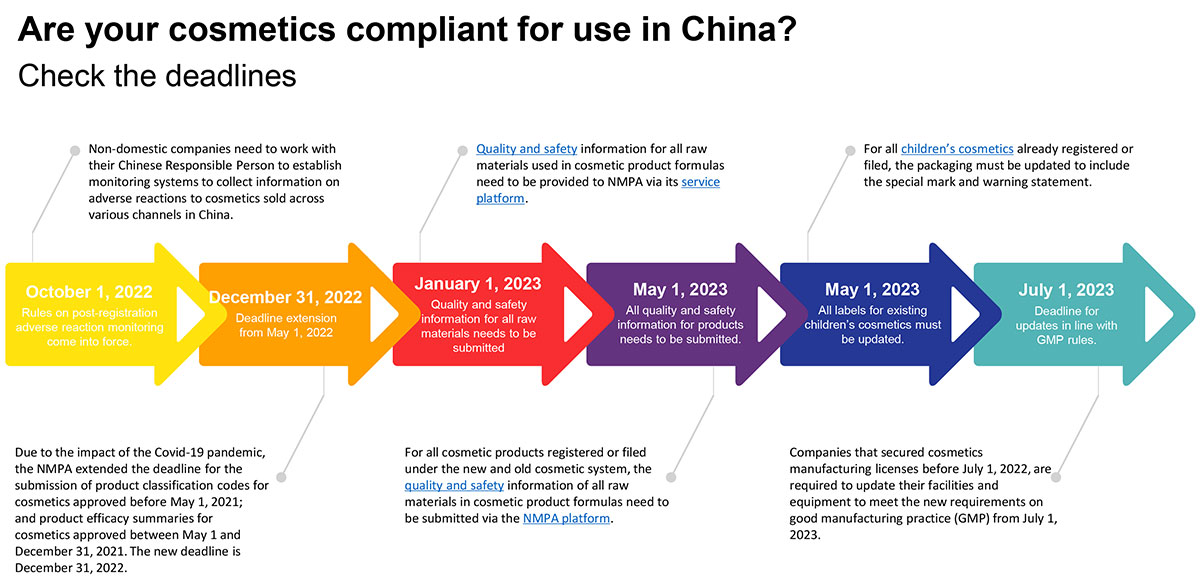
To help you keep up-to-date and compliant we have prepared this infographic of the upcoming compliance deadlines.