Generally Recognized As Safe (GRAS) means that a substance is generally presumed to be safe. If a substance is recognized as safe under its intended use, it is classified as GRAS and can be exempted from pre-market approval procedures required by U.S. law. As a key basis for recognizing new food ingredients in the United States, FDA GRAS notifications remain active. After nearly three months without updates, the FDA recently made a significant update to the FDA GRAS list, with the most recent update being on June 10, 2025.
CIRS Group has conducted a detailed statistical analysis and summary of the FDA GRAS substances from January to June 2025, providing insights and references for companies.
Summary of the submission and approval of FDA GRAS Notices in the first half of 2025
On the FDA website, GRAS dossier statuses are categorized into three types:
- FDA has no questions;
- Pending; and
- At the notifier's request, FDA ceased to evaluate this notice.
In the January-June 2025 update, the status for a total of 59 substances was updated. Among them, 34 substances passed the certification and received the “FDA has no questions” status, 15 new substances were pending, and another 10 substances were no longer being evaluated.
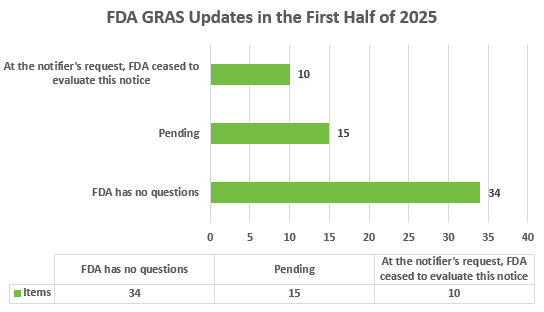
Figure 1. GRAS Notice Submitted to FDA in the First Half of 2025
Substances notified and updated on the FDA website in the first half of 2025 (based on publication date): a total of 34 substances
In the first and second quarters of 2025, 34 substances were updated on the FDA website as having received “FDA has no questions” status. Among them: 13 were microbial-derived substances, 12 were other types of substances, 5 were sugar substitute ingredients, 3 were dairy-based products, and 1 was a human milk oligosaccharide (HMO). These GRAS notifications were submitted by companies from a variety of countries, including the United States, China, South Korea, Japan, Canada, the Netherlands, Ireland, India, Estonia, and Chile. Notably, companies from the U.S. and China were the most active contributors.
Note: Although these 34 substances were only recently updated on the FDA website, many of them had actually received their “FDA has no questions” letters much earlier. The approval dates range widely from February 5, 2024, to March 14, 2025. A significant portion of the dossiers were approved between June and December 2024, while a few were approved in Q1 2025. The delayed publication on the FDA website accounts for the recent appearance of these approvals in the public database.
Table 1. Substances updated with “FDA has no questions” status in Q2 2025
S.N | GRN NO. | Name | Product Type | Company | Date |
1 | 1069 | others | Cargill, Inc. (US) | 2024.02.05 | |
2 | 1109 | β-agarase enzyme preparation produced by Streptomyces coelicolor | microbial-derived substances | Dyne Bio Inc. (Korea) | 2024.04.15 |
3 | 1117 | Mycelial biomass from Neurospora crassa | microbial-derived substances | The Better Meat Co. (US) | 2024.07.09 |
4 | 1120 | α-Galactosidase enzyme preparation produced by Aspergillus niger | microbial-derived substances | Novozymes North America (US) | 2024.06.05 |
5 | 1122 | others | NapiFeryn BioTech Sp. z o. o. (Poland) | 2024.07.25 | |
6 | 1126 | Calcium acetate | others | Niacet Corporation (US) | 2024.06.07 |
7 | 1129 | Heat-killed Clostridium tyrobutyricum strain ASM#19 | microbial-derived substances | Superbrewed Food, Inc. (US) | 2024.02.22 |
8 | 1133 | others | Anderson Global Group (US) | 2024.01.22 | |
9 | 1139 | Transglutaminase enzyme preparation produced by Bacillus licheniformis carrying a gene encoding transglutaminase from Streptomyces mobaraensis | microbial-derived substances | Novozymes North America (US) | 2024.07.02 |
10 | 1147 | others | Copperprotek SPA (Chile) | 2024.07.05 | |
11 | 1148 | D-psicose | sugar substitute ingredients | Daesang Corporation (Korea) | 2024.09.30 |
12 | 1151 | others | Cooperative Koninklijke Cosun U.A. (Netherlands) | 2024.06.12 | |
13 | 1154 | microbial-derived substances | Lallemand Inc. (Canada) | 2024.08.15 | |
14 | 1157 | 2'-fucosyllactose | HMO | Synaura Biotechnology (Shanghai) Co., Ltd.(China) | 2024.08.07 |
15 | 1159 | microbial-derived substances | Pellucid Lifesciences Pvt., Limited (India) | 2024.05.06 | |
16 | 1161 | Triacylglycerol lipase enzyme preparation produced by Candida cylindracea | microbial-derived substances | Meito Sangyo Co., Ltd. (Japan) | 2024.09.24 |
17 | 1164 | A chemically synthesized analog of pediocin PA-1 specific to Listeria monocytogenes | others | Innodal and Quality Systems Solutions (Canada) | 2024.08.07 |
18 | 1166 | Fava bean (Vicia faba L.) protein hydrolysate | others | Nuritas (Ireland) | 2024.11.13 |
19 | 1167 | sugar substitute ingredients | Perfect Day, Inc. (US) | 2024.10.08 | |
20 | 1170 | Resistant dextrin from tapioca in powder or syrup form | others | Icon Foods, Inc. (US) | 2024.10.22 |
21 | 1171 | Collagen polypeptide produced by Escherichia coli K-12 S9188 | others | Geltor, Inc. (US) | 2024.10.22 |
22 | 1174 | dairy products | The LittleOak Company (US) | 2024.12.06 | |
23 | 1175 | Lactiplantibacillus plantarum DSM 23881 | microbial-derived substances | Nordwise BioCC OÜ (Estonia) | 2024.10.22 |
24 | 1183 | Monellin preparation produced by Komagataella phaffii P-MON-040 expressing a gene encoding for a modified monellin | microbial-derived substances | Oobli, Inc. (US) | 2024.11.22 |
25 | 1184 | Rebaudioside M from a modified strain of Escherichia coli BL21(DE3) | sugar substitute ingredients | Sichuan Ingia Biosynthetic Co., Ltd. (China) | 2024.10.23 |
26 | 1187 | microbial-derived substances | AB Enzymes Inc. (US) | 2025.02.28 | |
27 | 1188 | D-psicose | sugar substitute ingredients | Shandong Starlight So True Biological Technology Co., Ltd (China) | 2024.11.19 |
28 | 1190 | microbial-derived substances | Omega Yeast Labs, LLC (US) | 2024.09.27 | |
29 | 1191 | Ergothioneine produced by Escherichia coli BL-21 (DE3) expressing ergothioneine synthases from Schizosaccharomyces pombe | others | Shanghai EGT Synbio Group Co., LTD (China) | 2025.01.30 |
30 | 1197 | microbial-derived substances | AB Enzymes Inc. (US) | 2025.03.14 | |
31 | 1198 | Inositol | others | Sichuan Bohaoda Biological Technology Co., Ltd. (China) | 2025.02.03 |
32 | 1206 | Rebaudioside M produced by enzymatic treatment of rebaudioside A purified from the leaves of Stevia rebaudiana (Bertoni) Bertoni | sugar substitute ingredients | Adorvia Biotechnology Co., Ltd. (China) | 2025.02.28 |
33 | 1211 | dairy products | Crossway Foods Limited (Ireland) | 2025.02.04 | |
34 | 1212 | dairy products | Crossway Foods Limited (Ireland) | 2025.02.07 |
Note: GRN 1187 is a resubmission of GRN 1110, and GRN 1197 is a resubmission of GRN 1121.
Substances ceased to be evaluated in the first half of 2025 and their reasons
As of June 10, 2025, a total of 10 GRAS notifications were marked as “At the notifier’s request, FDA ceased to evaluate this notice” in the FDA database during the first half of the year. Due to delays in the FDA’s website updates, most of these dossiers were actually withdrawn in 2024, but their status was not publicly updated until 2025. Details are summarized in Table 1 below.
Table 2. GRAS notifications marked as ceased to be evaluated in the first half of 2025
GRN NO. | Name | Product Type | Company | Date | Reasons |
1141 | L-α-glycerophosphorylcholine | others | Shenyang Gold Jyouki Technology Co., Ltd (China) | 2024.04.29 | Insufficient EDI data and safety data. |
1152 | Phoenix oyster mushroom (Pleurotus pulmonarius) mycelia biomass | others | Mushlabs GmbH (Germany) | 2024.03.21 | The lack of data on potential gene expression differences between the fruiting body and the mycelium raises concerns regarding differential safety profiles for human consumption. |
1153 | others | Terviva, Inc. (US) | 2024.05.23 | The substance exhibits pharmacological activity. | |
1160 | Lemna leaf protein | others | Plantible Foods (US) | 2024.05.06 | Key safety data are not publicly available. |
1163 | A preparation containing two bacteriophages (phage) specific to Salmonella enterica | microbial-derived substances | Cytophage Technologies inc. (Canada) | 2024.05.28 | Change in manufacturing process. |
1168 | Tigernuts flour | others | Tigernut Traders, SL (Spain) | 2024.08.06 | There are concerns regarding the reliability of the safety data. The extrapolation factor from animal studies lacks justification, and the toxicological study was conducted solely in female rats. |
1169 | HMO | GeneChem, Inc. (Korea) | 2024.10.07 | There is inadequate information on the removal of impurities such as lithium during production. The compositional data are not clearly presented, and the exposure assessment based on intended use lacks transparency and scientific rigor. | |
1176 | Lacto-N-tetraose | HMO | FrieslandCampina Ingredients B.V.(Netherlands) | 2024.09.24 | Deficiencies were identified in the compositional analysis of LNT, including the absence of impurity profiles and the use of cobalt in fermentation, which may pose risks if not properly controlled. |
1180 | Cellobiose | sugar substitute ingredients | SAVANNA Ingredients GmbH (Germany) | 2024.09.09 | The submission lacks sufficient detail on the manufacturing process, raises questions concerning the intended use and the accuracy of dietary exposure estimates, and does not provide adequate data on the safety and tolerability of cellobiose in infants. |
1194 | Partially hydrolyzed eggshell membrane powder derived from chicken eggs | others | ESM Technologies, LLC (US) | 2025.02.04 | The current safety data package is inadequate, with key gaps in information such as protein characterization, allergenicity assessment, accurate estimation of dietary exposure, and evaluation of long-term effects. Additionally, the intended use of the substance requires clarification, and potential arsenic content should be carefully addressed. |
GRAS notice by Chinese companies in the first half of 2025
In this update cycle, a total of nine GRAS notices submitted by Chinese companies had their statuses updated on the FDA website. Among them: 6 substances received “FDA has no questions” letters, 2 substances were new submissions currently pending and 1 substance was ceased to be evaluated. The substances involved include HMOs, sugar substitutes, microbial-derived substances, and other nutritional supplements.
Table 3. GRAS notifications submitted by Chinese companies that updated in the first half of 2025
SN | GRN NO. | Name | Product Type | Company | Status | Date |
1 | 1141 | L-α-glycerophosphorylcholine | others | Shenyang Gold Jyouki Technology Co., Ltd | At the notifier's request, FDA ceased to evaluate this notice | 2024.04.29 |
2 | 1157 | 2'-fucosyllactose | HMO | Synaura Biotechnology (Shanghai) Co., Ltd. | FDA has no questions | 2024.08.07 |
3 | 1184 | Rebaudioside M from a modified strain of Escherichia coli BL21(DE3) | sugar substitute ingredients | Sichuan Ingia Biosynthetic Co., Ltd. | FDA has no questions | 2024.10.23 |
4 | 1188 | D-psicose | sugar substitute ingredients | Shandong Starlight So True Biological Technology Co., Ltd | FDA has no questions | 2024.11.19 |
5 | 1191 | Ergothioneine produced by Escherichia coli BL-21 (DE3) expressing ergothioneine synthases from Schizosaccharomyces pombe | others | Shanghai EGT Synbio Group Co., LTD | FDA has no questions | 2025.01.30 |
6 | 1198 | Inositol | others | Sichuan Bohaoda Biological Technology Co., Ltd. | FDA has no questions | 2025.02.03 |
7 | 1206 | Rebaudioside M produced by enzymatic treatment of rebaudioside A purified from the leaves of Stevia rebaudiana (Bertoni) Bertoni | sugar substitute ingredients | Adorvia Biotechnology Co., Ltd. | FDA has no questions | 2025.02.28 |
8 | 1230 | others | Sichuan Ingia Biosynthetic Co., Ltd. | pending | / | |
9 | 1232 | microbial-derived substances | SunWay Biotech Co., Ltd. | pending | / |
Summary
In the first half of 2025, the FDA’s GRAS database updates primarily reflected the backlog of submissions from 2024, leading to a significant increase in the number of notified substances. Chinese companies remained highly active in GRAS notices, continuing to show strong performance in the sugar substitute category, while also expanding into areas such as HMOs, microbial-derived ingredients, and functional raw materials.
To improve the pass rate of GRAS notices, CIRS Group recommends that companies provide comprehensive safety assessment data, establish clear and reliable specifications, and calculate reasonable dietary exposure levels to ensure the safety of the substance. CIRS will continue to provide full-service support to help companies successfully navigate the GRAS notice process.
Data Source: FDA GRAS Inventory and most recently published GRAS notices (last updated on June 10, 2025).
Notes:
Since the FDA does not disclose dossier notified dates, the data on substances in pending are based primarily on the submission dates disclosed in the notices.
All data in this report are based on publicly available GRAS notices with assigned GRAS numbers and are for reference only.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.

