On May 7, CMDE issued a notice that the NMPA's medical device Electronic Regulated Product Submission System (eRPS System) will start the trial operation soon. In order to secure the user's account and data, the medical device applicant/registrant must log in to the Electronic Regulated Product Submission System (eRPS System) using the Certificate Authority (CA). From May 10th, medical device applicants/registrants can apply for a CA through the eRPS system. The CA claimant/holder need to be the applicant of Chinese Class III medical device companies or the agent of the imported medical device manufacturing enterprise. Each company is only allowed to apply for one CA certificate with a signature function.
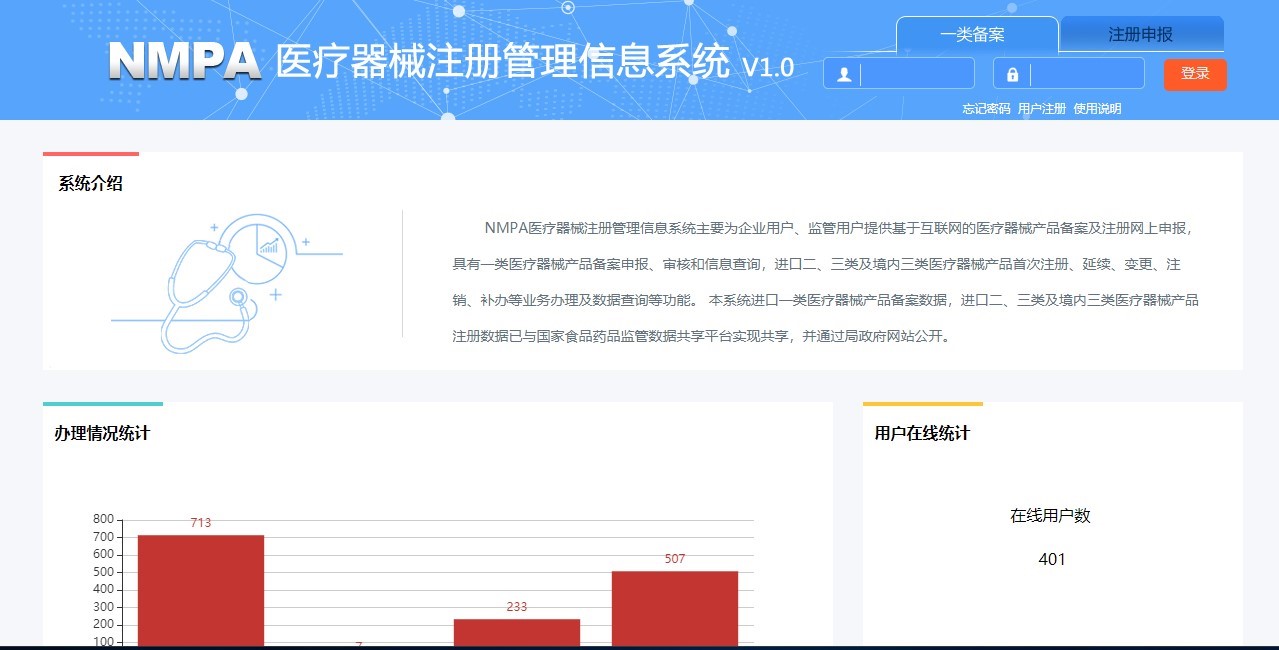
The NMPA's medical device Electronic Regulated Product Submission System (eRPS System) provides online-based medical device filing and registration services for enterprise users and regulatory users. It has Class I medical device product filing, imported Class II and III Medical device registration, certificate renewal, certificate alternation, cancellation and other services. A CMDE spokesperson said that the construction and promotion of the eRPS system will reduce the reporting burden of medical device administrative counterparts, improve the quality of registration materials, and optimize the evaluation process.
China Medical Device Regulation Related Resources provided by CIRS:
? China NMPA Medical Device Filing and Registration Consulting
? Clinical Trial Consulting
? Medical Device GMP System Assessment
? China Medical Device Regulation Training
? Guideline: Medical Device Registration in China
? Annual Report: Medical Device Market in China

