In the EU, all pesticides sold or used must be registered first before they can be placed on the EU market. The most important pesticide regulation is the Regulation (EC) No 1107/2009 (also known as Plant Protection Products Regulation, PPP), which lays down both rules for the approval of active substances (including micro-organisms), safeners and synergists, and the authorization of plant protection product formulations. Other non-crop protection pesticide products such as pest control, preservatives and disinfectants are usually regulated by the EU Biocidal Products Regulation (BPR, Regulation (EU) No. 528/2012).
Who Shall Register under EU PPP Regulation?
Pesticide manufacturers and/or importers in EU; or
Companies who export pesticides to the EU market
Pesticide Regulatory Authorities in EU
European Commission (EC)
Council of the European Union
European Parliament
European Food Safety Authority (EFSA)
European Chemicals Agency (ECHA)
Competent authorities of MS
Pesticide Registration Process in EU
The European Union implements a two-tiered regulatory process for pesticide approval and authorization: approval of active substance at the EU level first and then the authorization of plant protection products at the Member State level.
Approval of Active Substances in EU
For a new active substance, an applicant should submit a dossier with all required scientific data and studies (product chemistry, toxicology, environment, i.e.) to a Member State (called the Rapporteur Member State) to begin the review process of active substance approval at the EU level. A new active substance may be approved for a maximum of up to 15 years. Once approved, it is accepted in all Member States, the source of which will become the reference source.
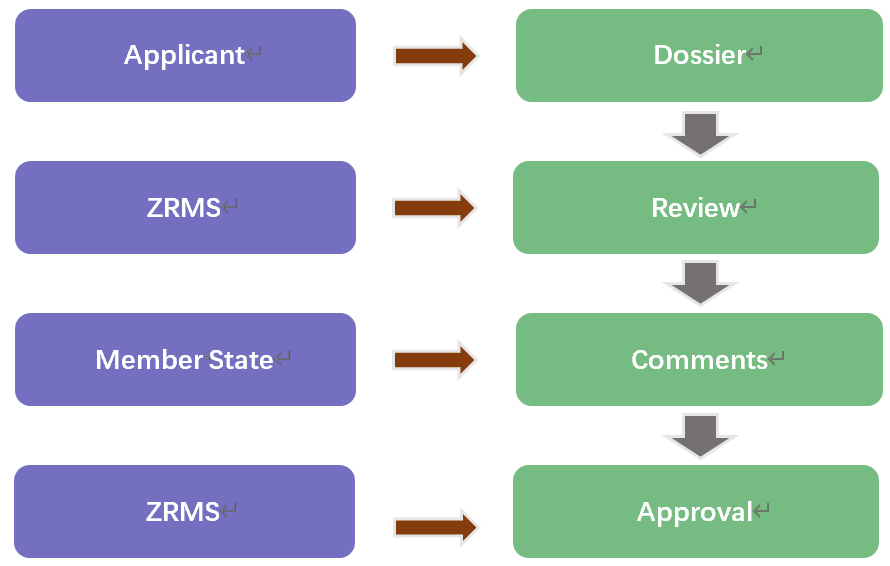
If you only export approved existing active substances to the EU, the easiest way to gain EU market access is to apply for technical equivalence assessment (TE), which assesses whether a new source of technical material is equivalent to a reference source. For TE, only limited data needs to be submitted. Once the TE is approved, it is valid in the entire EU Member States. More info about be found in our EU Technical Equivalence Assessment service.
Authorization of Plant Protection Products (PPP) in EU
Upon securing active substance approval, the authorization of Plant Protection Products (PPPs) proceeds through individual Member State regulatory processes. Authorization for a pesticide product must be granted by every Member State that the applicant wants to sell the product to.
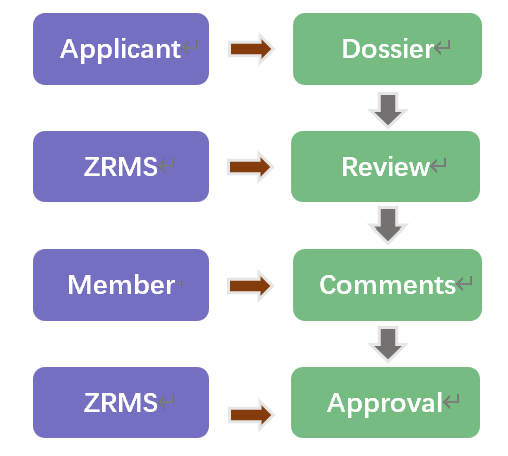
Authorizations granted by one Member State may be accepted by other Member States where agricultural, plant health and environmental (including climatic) conditions are comparable. This can be done by the application of Mutual Recognition.
The EU is divided into three Zones according to agricultural climate conditions:
Zone A: North (Denmark, Estonia, Latvia, Lithuania, Finland, Sweden);
Zone B: Central (Belgium, Czech Republic, Germany, Ireland, Luxembourg, Hungary, Netherlands, Austria, Poland, Romania, Slovenia, Slovakia, United Kingdom);
Zone C: South (Bulgaria, Greece, Spain, France, Italy, Cyprus, Malta, Portugal)
Our EU Pesticide Regulatory Services
Our experienced team at CIRS Group can help you navigate the complex pesticide regulatory barriers in the EU and bring your pesticide products to the EU market efficiently and compliantly.
Our services include:
Application of Technical Equivalence
Authorization of Plant Protection Products
Data Gap Analysis
GLP Study Monitoring Services
- Health and Environmental Risk Assessment
Dossier Preparation and Submission
EU Representation Services
For more information on how we can assist with your pesticide registration needs in EU, contact us today via Email: service@cirs-group.com.