Foods for Special Medical Purpose (FSMP) are made from special processing formula and used to meet the special dietary need for people who have limited feeding, digestion absorption disorders, metabolic disorders or specific disease condition, including the FSMP for infants 0-12 months of age and the FSMP for people over one year old.
In accordance with Food Safety Law of China (2015 version), FSMP products shall get registered with CFDA. According to the Notice on Transitional Period of FSMP Registration (No.139, 2017), since January 1 2019, all FSMP products produced in China or imported to China shall get the FSMP registration certificate under CFDA, and indicate the registration number on the label and instruction book.
Classification of FSMP
Classification | Specific Categories | |
|---|---|---|
FSMP for 0-12 months old infant | Lactose free formula or low lactose formula food | |
Partially hydrolyzed milk protein formula food | ||
Extensively hydrolyzed milk protein formula or amino acid-based formula food | ||
Premature or low birth weight infant formula food | ||
Breast milk nutrition food | ||
Amino acid metabolism disorder formula food | ||
FSMP for 1 year old and above | Full nutritional formula food | |
Specific full nutritional formula food | 1*. For diabetics; 2*. For respiratory system disease patients; 3*. For kidney disease patients; 4*. For cancer patients; 5. For hepatopaths; 6. For muscle attenuation syndrome patients; 7. For patients with trauma, infection, surgery or other stress condition; 8*. For patients with inflammatory bowel disease; 9*. For food protein allergy sufferers; 10*. For patients with intractable epilepsy; 11. For patients with gastrointestinal disorders, pancreatitis; 12. For patients with fatty acid metabolism; 13*. For patients with obesity, fat reducing surgery. | |
Incomplete nutritional formula food | ||
*Currently, for specific full nutritional formula food, the adjustable nutrients indexes of 8 specific categories (No.1, 2(COPD), 3, 4, 8, 9, 10,13) are available according to Q&A of GB 29922-2013.
FSMP Laws and Regulations
Laws and Regulations | Issued Date | Implemented Date |
|---|---|---|
Food Safety Law of The People’s Republic of China (2015 version) | 2015.04.24 | 2015.10.01 |
Administrative Measure on FSMP Registration | 2016.03.10 | 2016.07.01 |
Dossier and Requirements of FSMP Registration (Trial) (2017 revision) | 2017.09.06 | 2017.09.06 |
Stability Research Requirements of FSMP (Trial) (2017 revision) | 2017.09.06 | 2017.09.06 |
Label and Instruction Book Manuscript Requirements of FSMP (Trial) | 2016.07.14 | 2016.07.14 |
On-site Inspection and Judgment Rules for Manufacturers of FSMP Registration (Trial) | 2016.07.14 | 2016.07.14 |
Clinical Trials Quality Control Specifications of FSMP (Trial) | 2016.11.01 | 2016.11.01 |
GB 29922-2013 General Rules of FSMP | 2013.12.26 | 2014.07.01 |
GB 25596-2010 General Rules of Infant Formula FSMP | 2010.12.21 | 2012.01.01 |
GB 29923-2013 Good manufacturing practice for FSMP | 2013.12.26 | 2015.01.01 |
(………………….) | - | - |
Applicant Qualification
According to the Administrative Measure on FSMP Registration, no matter for domestic or imported FSMP products,the applicants shall be actualmanufacturerswho have production capacity..
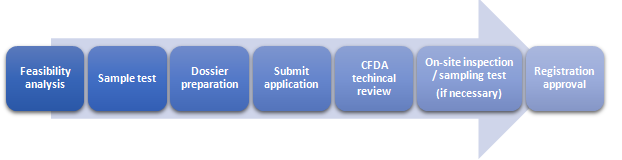
Registration Procedures
Dossier requirements
Application form
Products R&D reports, formula materials and formula design basis
Product process materials
Product standard requirements
Samples of product label and instruction book
Test reports
Evidence material of R&D capacity, production capacity and test capacity
Clinical trial reports (only required for specific full nutritional formula foods)
Application related certificates
For imported FSMP products, there are some additional certificates required:
Copy and Chinese version of qualification certifying document issued by government authorities or legal service agencies in the producing country (region) of origin proving that the applicant is the oversea manufacturer;
Copy and Chinese version of certifying document issued by government authorities or legal service agencies in the producing country (region) of origin proving that the product is allowed to be sold on the market. If the product is not available for sale, this document can be exempted.
For registration affairs run by oversea manufacturer’s Permanent Representative in China, a copy of the "registration certificate of oversea enterprise’s permanent Chinese representative offices" shall be provided;
For registration affairs run by domestic agencies entrusted by oversea manufacturers, the applicant shall provide the original notarized certificate of entrustment and its Chinese version, and copy of business license of the agency entrusted.
Test Items
The following tests are required for FSMP registration in China:
1) Tests of all items listed in product standard;
2) Stability tests**;
3) Clinical trials***;
4) Other tests if necessary.
Note:
**. For specific full nutritional formula food and part nutritional formula food, the stability test reports shall be submitted, but for full nutritional formula food andFSMP for 0-12 months of age, the applicant can keep the records for further examination.
***The clinical trials are ONLY required for specific full nutritional formula food.
Our Services
CIRS is providing one-stop services of China food regulatory compliance. For FSMP, we offer the following services:
1. Training on FSMP Regulation
2. FSMP Regulation Update Monitoring
3. FSMP Registration
4. Single Technology Services
Pre-market Investigation
Classification Analysis and Formula Review
Chinese Label and Package Insert Design
Translation
Test Arrange and Monitoring
Other customized service