In recent years, the enthusiasm for GRAS Notices to the U.S. FDA has remained strong. From July 2025 to the present, the FDA has released a large-scale update to the GRAS inventory. CIRS Group has conducted a detailed analysis and summary of GRAS submissions from July to September 2025, to help enterprises stay informed and make strategic reference.
It is important to note that updates on the FDA website are often delayed – many substances may have already completed the GRAS review and received “no questions” letters, but their public status remains unchanged. CIRS Group closely monitors FDA updates, and this report summarizes the information newly released in Q3 2025.
Overview of FDA GRAS Updates in Q3 2025
During the third quarter, four types of status appeared in FDA’s updates. In addition to the regular categories:
- FDA has no questions,
- Pending, and
- At the notifier’s request, FDA ceased to evaluate this notice.
A new status appeared:
- Notice does not provide a basis for a GRAS determination.
In total, 82 substances had status updates in Q3 2025: 47 “no questions” determinations, 32 new pending cases, two cases deemed insufficient for GRAS determination, and one case was ceased to evaluate by the notifier.
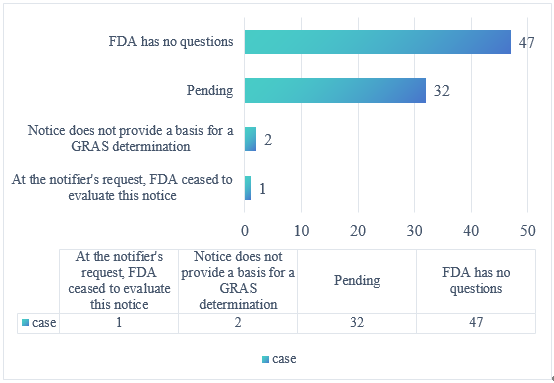
Figure 1. FDA GRAS Submission Status in the Third Quarter of 2025 (Based on FDA Website Updates)
Among these 82 substances, 31 were microorganism-related, 10 were HMOs (Human Milk Oligosaccharides), six were alternative sweeteners, one was a dairy product, and 34 belonged to other categories.
Microorganism-related materials remain a major focus, with numerous bacterial strains submitted, such as Saccharomyces cerevisiae, Lactiplantibacillus plantarum, Lacticaseibacillus rhamnosus, Bacillus velezensis, Bifidobacterium animalis, and Bifidobacterium animalis subsp. lactis. Enzymes and mycelial biomass products were also key parts of microorganism-related GRAS filings.
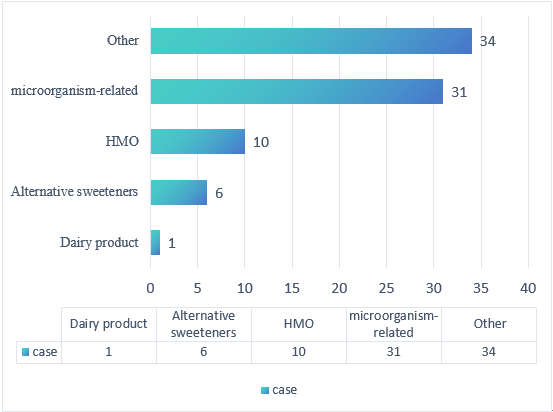
Figure 2. Number of Substances by Category in FDA GRAS Updates for the Third Quarter of 2025
Substances Granted “FDA Has No Questions” Status in Q3 2025: 47 Total (per FDA Update Date)
In Q3 2025, 47 substances were updated to “FDA has no questions”. Among these, 19 were microorganism-related, four were alternative sweeteners, two were HMOs, one was a dairy product, and 21 fell under other categories. Applicants came from a diverse range of countries, including the United States, China, South Korea, Japan, Canada, the Netherlands, India, Australia, and Denmark. U.S. and Chinese companies were the two main contributors, accounting for 20 and 15 of the approved substances, respectively.
Comparison between FDA update dates and the actual issuance of “no questions” letters shows that nearly all entries experienced a publication delay, in some cases exceeding one year (e.g., GRN 1173).
Table 1. Substances Updated to “FDA Has No Questions” Status on the FDA Website in the Third Quarter of 2025
No. | GRN No. | Name | Company | Date |
1 | 1173 | Invertase enzyme preparation produced by Trichoderma reesei expressing a gene for invertase from Aspergillus niger | AB Enzymes Inc (US) | Aug 15, 2024 |
2 | 1177 | Unique Biotech Limited, India (India) | Jul 5, 2024 | |
3 | 1178 | Sichuan Ingia Biosynthetic Co., Ltd (China) | Aug 16, 2024 | |
4 | 1181 | Weizmannia coagulans LMG S-31876 spore preparation | Abode Biotec India Private Limited (India) | Jul 25, 2024 |
5 | 1182 | Hangzhou Viablife Biotech Co, Ltd. (China) | Feb 12, 2025 | |
6 | 1189 | Dry whole goat milk | Bubs Australia Limited (Australia) | Sep 24, 2024 |
7 | 1192 | Mitsubishi Chemical Corporation (Japan) | Mar 14, 2025 | |
8 | 1193 | D-psicose | Sichuan Ingia Biosynthetic Co., Ltd. (China) | Dec 12, 2024 |
9 | 1195 | Chymosin enzyme preparation produced by Aspergillus niger expressing a gene encoding chymosin from Erinaceus europaeus | Chr. Hansen A/S (Denmark) | Mar 6, 2025 |
10 | 1196 | AB Enzymes Inc. (US) | Mar 3, 2025 | |
11 | 1199 | Rhamnogalacturonan-I | G3P Inc. (US) | Feb 28, 2025 |
12 | 1200 | β-lactoglobulin produced by Komagataella phaffii strain VIPLA | Vivici B.V. (Netherlands) | Feb 28, 2025 |
13 | 1201 | Lipase enzyme preparation produced by Komagataella phaffii expressing a gene encoding lipase from Yarrowia lipolytica | Chr. Hansen A/S (Denmark) | Mar 10, 2025 |
14 | 1202 | Soy leghemoglobin preparation from a strain of Komagataella phaffii | Impossible Foods Inc (US) | Mar 7, 2025 |
15 | 1203 | Guilin Layn Natural Ingredients Corp. (China) | Feb 12, 2025 | |
16 | 1204 | Beijing Scitop Bio-tech Co., LTD (China) | Feb 19, 2025 | |
17 | 1205 | Kaneka Americas Holding, Inc. (US) | Mar 24, 2025 | |
18 | 1207 | Nanjing Bestzyme Bio-Engineering Co. Ltd. (China) | Apr 10, 2025 | |
19 | 1208 | Shandong Henglu Biotechnology Co., Ltd. (China) | Apr 29, 2025 | |
20 | 1210 | Glucosyltransferase enzyme preparation produced by Bacillus subtilis expressing the gene encoding glucosyltransferase from Streptococcus salivarius | Danisco US Inc. (US) | Aug 15, 2025 |
21 | 1213 | Crossway Foods Limited (Ireland) | Feb 21, 2025 | |
22 | 1214 | Unique Biotech Limited (India) | May 7, 2025 | |
23 | 1215 | Bacteriophage (phage) preparation specific to Listeria monocytogenes | FINK Tec GmbH (Germany) | Jun 6, 2025 |
24 | 1218 | Microbial Discovery Group LLC (US) | Apr 29, 2025 | |
25 | 1219 | TurtleTree Inc (US) | May 7, 2025 | |
26 | 1221 | Microbial Discovery Group, LLC (US) | Jun 9, 2025 | |
27 | 1222 | Suntory Global Spirits (US) | May 5, 2025 | |
28 | 1223 | Suntory Global Spirits (US) | Jun 3, 2025 | |
29 | 1224 | Suntory Global Spirits (US) | Jun 3, 2025 | |
30 | 1225 | Suntory Global Spirits (US) | Jun 3, 2025 | |
31 | 1230 | Vanillin preparation produced by Escherichia coli BL21(DE3) SI-VAN1 | Sichuan Ingia Biosynthetic Co., Ltd. (China) | Jun 11, 2025 |
32 | 1231 | Bacillus velezensis PTA-127359 | BIO-CAT Microbials, LLC (US) | Jul 11, 2025 |
33 | 1233 | Sansho (Zanthoxylum piperitum) pepper distillate | Suntory Global Spirits (US) | Jul 8, 2025 |
34 | 1234 | Suntory Global Spirits (US) | Jul 14, 2025 | |
35 | 1235 | Bioflag Co., Ltd (China) | Sep 2, 2025 | |
36 | 1236 | Algal oil (≥40% docosahexaenoic acid) from Aurantiochytrium limacinum H Sc-01 | Shaanxi Healthful Bioengineering Co., Ltd. (China) | Jun 10, 2025 |
37 | 1238 | 2'-fucosyllactose | Cataya Bio (Shanghai) Co., Ltd. (China) | Jun 17, 2025 |
38 | 1239 | Wecare Probiotics Co., Ltd (China) | Jun 11, 2025 | |
39 | 1240 | Wecare Probiotics Co., Ltd (China) | Jul 8, 2025 | |
40 | 1242 | BENEO GmbH (Germany) | Jul 14, 2025 | |
41 | 1243 | Bacillus coagulans M2017813 spore preparation | Thankcome Biological Science and Technology Co., Ltd. (China) | Aug 21, 2025 |
42 | 1244 | NORDWISE BioTech OÜ (US) | Aug 5, 2025 | |
43 | 1245 | NORDWISE BioTech OÜ (US) | Aug 5, 2025 | |
44 | 1246 | Berkeley Fermentation Science Inc. (US) | Sep 22, 2025 | |
45 | 1247 | β-Lactoglobulin produced by Kluyveromyces lactis CCTCC M20241460 | Shanghai Changing Biotechnology Co., Ltd. (China) | Sep 19, 2025 |
46 | 1248 | Yeast biomass produced by Kluyveromyces marxianus CCTCC M 20211265 | Shanghai Changing Biotechnology Co., Ltd. (China) | Sep 23, 2025 |
47 | 1249 | Onego Bio, Inc. (US) | Sep 16, 2025 |
Substances from Chinese Companies Granted “FDA Has No Questions” Status in Q3 2025: 15 Total (per FDA Update Date)
In Q3 2025, 15 substances from Chinese enterprises were granted the “FDA has no questions” status: five microorganism-related (including four bacterial strains and one yeast biomass), four alternative sweeteners, two HMOs, and four others. The sweeteners covered three of the most popular categories: rebaudiosides, D-allulose, and brazzein. The two HMOs were lacto-N-triose and 2’-fucosyllactose, both leading products in precision nutrition.
Table 2. Substances from Chinese Enterprises Granted “FDA Has No Questions” Status in the Third Quarter of 2025
No. | GRN No. | Substance Name | Company | Date of Completion |
1 | 1178 | Sichuan Ingia Biosynthetic Co., Ltd (China) | Aug 16, 2024 | |
2 | 1182 | Hangzhou Viablife Biotech Co, Ltd. (China) | Feb 12, 2025 | |
3 | 1193 | D-psicose | Sichuan Ingia Biosynthetic Co., Ltd. (China) | Dec 12, 2024 |
4 | 1203 | Guilin Layn Natural Ingredients Corp. (China) | Feb 12, 2025 | |
5 | 1204 | Beijing Scitop Bio-tech Co., LTD (China) | Feb 19, 2025 | |
6 | 1207 | Nanjing Bestzyme Bio-Engineering Co. Ltd. (China) | Apr 10, 2025 | |
7 | 1208 | Shandong Henglu Biotechnology Co., Ltd. (China) | Apr 29, 2025 | |
8 | 1230 | Vanillin preparation produced by Escherichia coli BL21(DE3) SI-VAN1 | Sichuan Ingia Biosynthetic Co., Ltd. (China) | Jun 11, 2025 |
9 | 1235 | Bioflag Co., Ltd (China) | Sep 2, 2025 | |
10 | 1236 | Algal oil (≥40% docosahexaenoic acid) from Aurantiochytrium limacinum H Sc-01 | Shaanxi Healthful Bioengineering Co., Ltd. (China) | Jun 10, 2025 |
11 | 1238 | 2'-fucosyllactose | Cataya Bio (Shanghai) Co., Ltd. (China) | Jun 17, 2025 |
12 | 1240 | Wecare Probiotics Co., Ltd (China) | Jul 8, 2025 | |
13 | 1243 | Bacillus coagulans M2017813 spore preparation | Thankcome Biological Science and Technology Co., Ltd. (China) | Aug 21, 2025 |
14 | 1247 | β-Lactoglobulin produced by Kluyveromyces lactis CCTCC M20241460 | Shanghai Changing Biotechnology Co., Ltd. (China) | Sep 19, 2025 |
15 | 1248 | Yeast biomass produced by Kluyveromyces marxianus CCTCC M 20211265 | Shanghai Changing Biotechnology Co., Ltd. (China) | Sep 23, 2025 |
Substances from Chinese Companies Newly Under Review in Q3 2025: 14 Total (per FDA Update Date)
According to FDA’s latest updates, 14 new substances from Chinese enterprises entered the review process in Q3 2025. Six were HMOs, including 2’-fucosyllactose, lacto-N-neotetraose, lacto-N-tetraose, and 6’-sialyllactose sodium salt. Five were microorganism-related, including four bacterial strains and one mycelial biomass product. The remaining three were lycopene preparations, fungal oil, and L-ergothioneine.
Table 3. Substances from Chinese Enterprises Newly Under Review in the Third Quarter of 2025
No. | GRN No. | Name | Company |
1 | 1253 | Lycopene preparation produced by Saccharomyces cerevisiae JZL03 | Wuhan Hesheng Technology Co., Ltd. |
2 | 1255 | MoreMeat (Guangzhou) Biotech Co., Ltd | |
3 | 1259 | Fungal oil (≥40% arachidonic acid (ARA)) from Mortierella alpina strain TKA-1 | ATK Biotech Co., Ltd. |
4 | 1261 | 2'-fucosyllactose | Suzhou Yixi Biotech Co., Ltd. |
5 | 1262 | 2'-fucosyllactose | Zhuhai Long Health Biotechnology Co., Ltd. |
6 | 1264 | Beijing Scitop Bio-Tech Co., Ltd. | |
7 | 1270 | L-ergothioneine produced by Escherichia coli K-12 MG1655 expressing enzymes from Neurospora crassa and Mycolicibacterium smegmatis MC2 155 | Gene III Biotechnology Co., Ltd. |
8 | 1272 | Lacto-N-neotetraose | Shenzhen Long Health Biotechnology Co., Ltd. |
9 | 1273 | Shenzhen Long Health Biotechnology Co., Ltd. | |
10 | 1274 | 2'-fucosyllactose | Tianjin Hesheng Biotechnology Co., Ltd. |
11 | 1275 | 6′-sialyllactose sodium salt | Cataya Bio (Shanghai) Co., Ltd. |
12 | 1277 | ReviveBio, Co. | |
13 | 1278 | Beijing Scitop Bio-Tech Co., Ltd. | |
14 | 1279 | Angel Yeast Co., Ltd. |
Summary
In the third quarter of 2025, the U.S. FDA GRAS list experienced a significant wave of updates. Microorganism-related substances (31 total) remained the hottest submission category, covering a broad range of probiotics, enzyme preparations, and emerging mycelial biomass materials. HMOs and alternative sweeteners also maintained high activity levels, reflecting the ongoing market drive for precision nutrition and sugar reduction. Chinese biotechnology companies stood out as key players this quarter, with 15 substances receiving FDA “no questions” letters (based on update timing) and 14 new filings in pending.
CIRS Group’s U.S. subsidiary and full-time regulatory experts provide professional support for GRAS submissions, leveraging extensive international experience to help enterprises accelerate approvals and enhance compliance. For consultation or collaboration, please feel free to contact or visit us.
Notes:
Data source: FDA official GRAS inventory and newly published GRAS notices (as of September 30, 2025).
As FDA does not disclose exact acceptance dates, statistics for “pending” substances are primarily based on the submission dates stated in public GRAS notices.
All data are based on officially accepted and published GRAS notices with identifiable GRAS numbers and are provided for reference only.
About CIRS
The Food Division of CIRS Group was established in 2012 and has a professional team specializing in US GRAS notices. The Food Division has extensive experience in various fields, covering GRAS, new food ingredients, new food additives, food contact materials, synthetic biology foods, EU Novel Foods, dietary supplements, and special dietary foods.
CIRS operates a fully-owned subsidiary in the US. By leveraging the expertise of the CIRS USA and the international teams, it can provide enterprises with various US food services, including but not limited to:
- US FDA GRAS Notice;
- US FDA Dietary Supplement Structure/Function Claim Notification;
- US New Dietary Ingredient (NDI) Notification;
- US FDA Registration of Food Facilities; and
- US Food Label/Advertisement Information Review.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.

