On November 18, 2025, China's National Center for Food Safety Risk Assessment (CFSA) issued eight new food raw materials. Comments are welcome before December 18, 2025. Details are as follows:
1. Peony seed oil
Name | Peony seed oil | |
Basic information | Source: Seeds of Paeoniaostii T. Hong & J. X. Zhang and Paeonia rockii (S. G. Haw& Lauener) T. Hong & J. J. Li | |
Production process | Made from Seeds of Paeoniaostii T. Hong & J. X. Zhang and Paeonia rockii (S. G. Haw& Lauener) T. Hong & J. J. Li, and produced through processes such as pressing, decolorization, and deodorization. | |
Quality specifications | Characteristics | Golden-yellow, transparent oily liquid. |
Fatty acid composition (as a percentage of total fatty acids) | ||
Oleic acid (C18:1), % | ≥21.0 | |
Linoleic acid (C18:2), % | ≥25.0 | |
Alpha-linolenic acid (C18:3), % | ≥38.0 | |
Note | 1. The scope of use does not include infant foods. 2. Food safety indicators shall comply with the current national food safety standards of China for vegetable oils and fats. 3. The information on peony seed oil in the former Ministry of Health Announcement No. 9 of 2011 is now obsolete. | |
2. Nekemias grossedentata leaf polyphenols
Name | Nekemias grossedentata leaf polyphenols |
Basic information | Source: Leaves of Nekemias grossedentata (Hand.-Mazz.) J. Wen& Z. L. Nie |
Production process | Made from Leaves of Nekemias grossedentata (Hand.-Mazz.) J. Wen& Z. L. Nie, and produced through processes such asethanol extraction, decolorization, concentration, filtration and drying. |
Recommend intake | ≤470 mg/day (calculated based on a total polyphenol content of 85 g/100 g; if the content exceeds this value, it should be converted according to the actual content). |
Note | 1. Scope of use and maximum dosage: - dairy and dairy products: modified milk and flavored fermented milk (0.5 g/kg), modified milk powder calculated based on the reconstituted liquid mass, cheese, processed cheese, cheese products, and condensed milk calculated based on the multiplication factor of raw milk. - beverages: liquid beverages ≤50 mL packaging (5 g/kg), 51–500 mL packaging (0.5 g/kg), and solid beverages calculated based on the reconstituted liquid mass. - jelly: 8 g/kg. - cocoa products, chocolate, and chocolate products(including cocoa butter substitute products): 8 g/kg. - candy: 25 g/kg. - frozen desserts: 5 g/kg. - alcoholic beverages: 2.5 g/kg. - preserved fruits: 5 g/kg. 2. Infants, pregnant women, and breastfeeding women should avoid consumption. Labels and instructions must clearly indicate the unsuitable populations and consumption limits. 3. Quality standards and food safety indicators refer to the appendix. |
3. Cyclocarya paliurus leaf polyphenols
Name | Cyclocarya paliurus leaf polyphenols |
Basic information | Structure form: Leaves from Cyclocarya paliurus (Batalin) Iljinsk. |
Production process | Made from leaves from Cyclocarya paliurus (Batalin) Iljinsk. through processes including hot water extraction, filtration, concentration, and drying. |
Recommend intake | ≤3 g/day |
Note | 1. Scope of use and maximum dosage: - dairy and dairy products: modified milk and flavored fermented milk (3 g/kg), modified milk powder calculated based on the reconstituted liquid mass, cheese, processed cheese, cheese products, and condensed milk calculated based on the multiplication factor of raw milk. - beverages: liquid beverages ≤50 mL packaging (30 g/kg), 51–500 mL packaging (3 g/kg), and solid beverages calculated based on the reconstituted liquid mass. - jelly: 48 g/kg. - cocoa products, chocolate, and chocolate products(including cocoa butter substitute products): 48 g/kg. - candy: 60 g/kg. - frozen desserts: 30 g/kg. - alcoholic beverages: 15 g/kg. - preserved fruits: 30 g/kg. 2. Infants, pregnant women, and breastfeeding women should avoid consumption. Labels and instructions must clearly indicate the unsuitable populations and consumption limits. 3. Quality standards and food safety indicators refer to the appendix. |
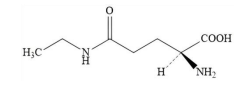
4. L-Theanine (fermentation)
Name | L-Theanine (fermentation) |
Basic information | Structure formula: CAS No.: 3081-61-6 Molecular formula: C7H14N2O3 Relative molecular mass: 174.20 |
Production process | Made from glucose, xylose, and other raw materials, through fermentation with Escherichia coli TH5-1, followed by filtration, decolorization, concentration, crystallization, and drying. Information on the production strain used in this process is provided in the appendix. |
Recommend intake | ≤250 mg/day |
Note | 1. Scope of use and maximum dosage: - dairy and dairy products: modified milk and flavored fermented milk (0.25 g/kg), modified milk powder calculated based on the reconstituted liquid mass, cheese, processed cheese, cheese products, and condensed milk calculated based on the multiplication factor of raw milk. - beverages: liquid beverages ≤50 mL packaging (2.5 g/kg), 51–500 mL packaging (0.25 g/kg), and solid beverages calculated based on the reconstituted liquid mass. - baked food: 2.5 g/kg. - cocoa products, chocolate, and chocolate products(including cocoa butter substitute products): 5 g/kg. - candy: 12.5 g/kg. 2. Infants, pregnant women, and breastfeeding women should avoid consumption. Labels and instructions must clearly indicate the unsuitable populations and consumption limits. 3. Quality standards and food safety indicators refer to the appendix. |
5. Bifidobacterium animalis subsp. lactis XLTG11
Name | Bifidobacterium animalis subsp. lactis XLTG11 |
Note | 1. Approved for inclusion in the “List of Microorganisms Permitted for Infant Foods”. 2. Food safety indicators shall comply with the “National Food Safety Standard for Microbial Preparations Used in Food Processing” (GB 31639), and Cronobacter spp. must not be detected (per 100 g). |
6. Mycoprotein from Fusarium compactum
Name | Mycoprotein from Fusarium compactum |
Production process | Produced using Fusarium compactum strain MM-135 as the production microorganism, through processes such as fermentation, nucleic acid removal, inactivation, and filtration. |
Note | 1. Not suitable for infants, young children, pregnant or breastfeeding women, and individuals allergic to edible fungi; the label and instructions should indicate the unsuitable population. 2. Quality specifications and food safety indicators are provided in the appendix. |
7. Auxenochlorella pyrenoidosa
Name | Auxenochlorella pyrenoidosa | |
Basic information | Genus and species: Chlorella genus, Chlorellaceae family | |
Production process | Produced from Chlorella vulgaris through processes such as cultivation, centrifugation, washing, separation, and drying. | |
Recommend intake | ≤20 g/day | |
Quality specifications | Characteristics | Light yellow to dark green powder or granular protein |
Protein content | ≥35.0 g/100 g | |
Moisture | ≤7.0 g/100 g | |
Ash | ≤7.0 g/100 g | |
Note | 1. Use does not include infant foods. 2. Food safety indicators shall comply with the provisions for algae and their products in the current national food safety standards of China. 3. The information on Chlorella vulgaris in the former Ministry of Health Announcement No. 19 of 2012 is now obsolete. | |
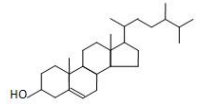
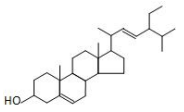
8. Plant sterol
Name | Plant sterol | |||
Basic information | Source: soybean oil, rapeseed oil, corn oil, sunflower oil, taro oil. 1. β-Sitosterol structural formula:
Molecular formula: C29H50O Relative molecular mass: 414.71 2. Campesterol structural formula:
Molecular formula: C28H48O Relative molecular mass: 400.66 3. Stigmasterol (or Soy sterol) structural formula:
Molecular formula: C29H48O Relative molecular mass: 412.69 | |||
Production process | Process 1: Made from fractions of soybean oil and other vegetable oils or taro oil, through saponification, extraction, and crystallization. Process 2: Made from fractions of soybean oil and other vegetable oils, through esterification, distillation, and crystallization. | |||
Recommend intake | ≤2.4 g/day | |||
Quality specifications | Characteristics | White to off-white powder or granules | ||
Plant sterols | ≥90 % | |||
Composition ratio of plant sterols | ||||
Source from soybean oil | Source from taro oil | |||
β-Sitosterol | ≥30.0% | β-Sitosterol | ≥70.0% | |
Campesterol | ≥15.0% | Campesterol | ≥4.0% | |
Stigmasterol (Soy sterol) | ≥12.0% | Stigmasterol (Soy sterol) | ≤2.0% | |
Note | 1. Use does not include infant foods. 2. The information on plant sterols in the former Ministry of Health Announcement No. 3 of 2010 is now obsolete. 3. Food safety indicators must comply with the following requirements: | |||
Lead (Pb): | ≤0.1 mg/kg | |||
Total arsenic (As) | ≤0.1 mg/kg | |||
Benzo[a]pyrene | ≤10 μg/kg | |||
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further information