On March 21, 2025, China National Center for Food Safety Risk Assessment (CFSA) issued four new food materials. Comments are welcomed before April 20, 2025.

Details are as follows:
1. Lutein esters
Name | Lutein esters | ||
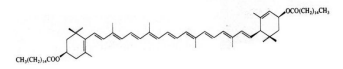
Basic information | Source: Tagetes erecta L. Structure formula: CAS No.:547-17-1 Molecular formula: C72H116O4 relative molecular mass: 1045.71 | ||
Production process | Made from Tagetes erecta L. as the raw material, it is processed through dehydration and crushing, solvent extraction, low molecular weight alcohol purification, and vacuum concentration. | ||
Recommended level | ≤ 36 mg/day (calculated based on Helenien content). | ||
Quality specifications | Characteristics | Dark reddish brown fine particles | |
Helenien, g/100g | ≥ 55.8 (See Appendix A for test methods) | ||
Zeaxanthin, g/100g | ≤ 4.2 (See Appendix A for test methods) | ||
Note | 1. Scope of use: bakery products, dairy products, beverages, ready-to-eat cereals, frozen beverages, condiments and confectionery, excluding foods for infants and young children. 2. The information related to Helenien in the original Ministry of Health Announcement No. 12 of 2008 is abolished. 3. Quality standards and food safety indicators refer to the appendix. | ||
Hexane, mg/kg | ≤10.0 | ||
Pb, mg/kg | ≤1.0 | ||
Cd, mg/kg | ≤0.5 | ||
Hg, mg/kg | ≤0.1 | ||
As, mg/kg | ≤1.0 | ||
Benzo[a]pyrene, μg/kg | ≤2.0 | ||
Total colony, CFU/g | ≤1000 | ||
Coliform, CFU/g | ≤10 | ||
Mold and yeast, CFU/g | ≤100 | ||
Salmonella, /25 g | ND | ||
Staphylococcus aureus, /25 g | ND | ||
Listeria monocytogenes, /25 g | ND | ||
2. D-Psicose/D-Allulose
Name | D-Psicose/D-Allulose |
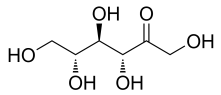
Basic information | Structure formula:
CAS No.:551-68-8 Molecular formula: C6H12O6 relative molecular mass: 180.16 |
Production process | Process 1: Made from glucose or sucrose by fermentation, purification, and drying of Escherichia coli AS10. Process 2: Made from fructose as a raw material, catalyzed by permitted D-psicose 3-epimerase, and then made through the process of decolorization, separation, purification, and crystallization. |
Recommended level | ≤ 20 g/day |
Note | 1. Should not be consumed by infants and young children, pregnant women, and breastfeeding women. Labeling, instructions should be marked unsuitable for people and consumption limits. 2. Quality specifications and food safety indicators are shown in the appendix. |
3. Bifidobacterium animalis subsp.lactis BLa80
Name | Bifidobacterium animalis subsp.lactis BLa80 |
Note | 1. Approved to be included in the List of Strains that can be used in foods for Infants and Young Children. 2. The food safety indicators shall comply with the National Standard for Food Safety Strain Preparations for Food Processing (GB31639), while Cronobacter spp. shall not be detected (/100 g). |
4. Bifidobacterium longum subsp. infantis LMG11588
Name | Bifidobacterium longum subsp. infantis LMG11588 |
Note | 1. Approved to be included in the List of Strains that can be used in foods for Infants and Young Children. 2. The food safety indicators shall comply with the National Standard for Food Safety Strain Preparations for Food Processing (GB31639), while Cronobacter spp. shall not be detected (/100 g). |
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further information