On March 1, 2024, China National Center for Food Safety Risk Assessment (CFSA) issued 3 new food raw materials, namely maritime pine bark extract, Bifidobacterium longum subsp. infantis M-63 and N-acetylglucosamine for public comments. Comments are welcomed before April 1, 2024. Details are as follows:
Maritime pine bark extract
Name | Maritime pine bark extract | |
Basic information | Source: Pinus pinaster Aiton | |
Brief introduction of the production process | Prepared through grinding, extraction, filtration, concentration, drying and other processes using maritime pine bark extract as the raw material. | |
Recommended intake | ≤ 150 mg/day | |
Quality requirements | Property | Reddish brown powder |
Proanthocyanidines, g/100 g ≥ | 50.0 | |
Moisture, g/100 g ≤ | 8.0 | |
Ash, g/100 g ≤ | 0.7 | |
Other information | 1. Scope of use and maximum levels: Milk and milk products (0.2 g/kg for modified milk and flavored fermented milk, milk powder and the formulated products are converted according to the mass of liquid after preparation); beverages (0.08 g/kg for liquid beverages, solid beverages are converted according to the mass of liquid after preparation); jelly (1.4 g/kg); 2. The labels and instructions shall bear a statement indicating that they should not be consumed by infants, pregnant women and lactating women, along with the unsuitable group and the usage limit; and 3. Food safety indicators must comply with the following requirements: | |
Ethanol, mg/kg ≤ | 100 | |
Lead (Pb), mg/kg ≤ | 1.0 | |
Mercury (Hg), mg/kg ≤ | 1.0 | |
Arsenic (As), mg/kg ≤ | 0.1 | |
Aflatoxin B1, μg/kg ≤ | 10 | |
Colony count, CFU/g ≤ | 1000 | |
Coliforms, MPN/g ≤ | 0.92 | |
Mould and Yeast, CFU/g ≤ | 50 | |
Salmonella, /25g | 0 | |
Staphylococcus aureus, /25g | 0 | |
Bifidobacterium longum subsp. infantis M-63
Name | Bifidobacterium longum subsp. infantis M-63 |
Other information |
|
N-acetylglucosamine
Name | N-acetylglucosamine |
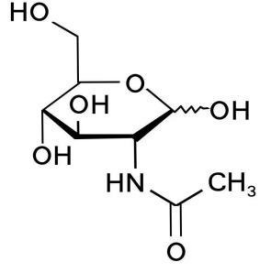
Basic information | Structure:
CAS number: 7512-17-6 Molecular formula: C8H15NO6 Molecular weight: 221.21 |
Brief introduction of the production process | Prepared through the fermentation of Corynebacterium glutamicum RDG-2110, followed by filtration, concentration, crystallization, centrifugation, alcohol washing, drying, and other processes using glucose, corn syrup powder, ammonium sulfate, potassium phosphate monobasic and magnesium sulfate as raw materials. |
Recommended intake | ≤500 mg/day |
Other information | 1. Scope of use and maximum levels: Milk and milk products (0.5 g/kg for modified milk and flavored fermented milk, milk powder and the formulated products are converted according to the mass of liquid after preparation); beverages (0.5 g/kg for liquid beverages, solid beverages are converted according to the mass of liquid after preparation); jelly (3 g/kg); candy (10 g/kg); cake (2 g/kg); 2. The labels and instructions shall bear a statement indicating that they should not be consumed by infants, pregnant women and lactating women, along with the unsuitable group and the usage limit; and 3. See the Appendix for quality specifications and food safety indicators. |
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further information