1. Background
On 2 May 2017, China Food and Drug Administration (CFDA) finally released all official supporting regulations of health food filing including Health Food Filing Guideline (Trial), Available Excipients for Health Food Filing and Their Usage Rules (Trial), and Main Production Processes of Health Food Filing Products (Trial). That is to say, the new policy-China health food filling has been officially initiated since May 2. In order to help applicants better understand the filing requirements, CIRS has made a comprehensive interpretation on these regulations and listed the highlights below.
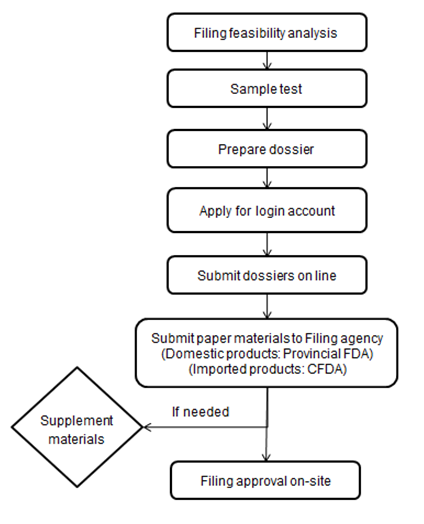
2. Health food filing procedure
3. Key points in filing material requirements
S.N. | Key points | Main requirements |
1 | Product formula materials | a) The varieties, dosages and standards of the used raw materials shall all comply with the Health Food Raw Materials Directory. Currently, the first batch is Directory of Health Function for Nutrition Supplement, which contains 22 vitamins & minerals, with 77 allowed compounds. b) For the excipients, they shall be compliant with the Available Excipients for Health Food Filing and Their Usage Rules (Trial) as well, which will be interpreted in details below. c) If the raw materials and excipients are pre-processed, all used materials shall be listed. |
2 | Production process materials | a) The main producing flow chart and detailed instructions including main process, key technology and technological parameters after the verification and conformation of pilot scale production shall be provided. b) It is not allowed to change the chemical structure and composition of the raw materials through reprocessing such as extraction or synthesis. |
3 | Safety and health function evaluation materials | a) Test reports of functional component, hygiene and stability for 3 batches samples pilot above level scales product shall be provided. b) If the applicant has test ability, the tests can be conducted by the applicant. If not, the tests can be entrusted to qualified lab. |
4 | Label and instruction manuscript | a) The indicating order of functional components shall be the same with the order in Health Food Raw Material Directory. b) For the product with 3 or above vitamins & minerals, the health function shall be claimed as “Supplement multi vitamins & minerals”. It is not allowed to claim as “Supplement (XXX raw materials) + (multi vitamins & minerals)”. In addition, for the product with 3 or above B vitamins, the health function can be claimed as “supplement B vitamins”. c) For the direction of nutrition supplement products, the total daily intake amount for solid preparation shall be ≤ 20g, and for liquid preparation shall be ≤ 30ml. d) The warning of “This product cannot replace drug. It is not suggested for people except the suitable crowed.” shall be added on the label. The warning of “Should not exceed the recommended amount or consume with same nutrients at the same time” shall be added for nutrition supplement product. Except above points, there are very detailed requirements on the labelling in the Guideline. Applicants shall pay attention to the label and instruction designation. |
5 | Product technical requirement materials | a) For the functional content of nutrition supplement product, the vitamin content range shall be 80%-180% of the labeled value, and the mineral content range shall be 75%-125% of the labeled value. b) The content range shall comply with the dosage requirements listed in the Health Food Raw Material Directory as well. |
6 | All items test reports | a) Applicant shall entrust legally qualified food test agency to carry out the all items test reports. b) 3 batches of all items tests according to the product technical requirements shall be conducted. Once the test report is issued, it cannot be changed. c) If the tests conducted in the same test agency of S.N. 3, the samples of them shall be different, and then there are 6 batches of samples required. |
4. Available Excipients for Health Food Filing
On 2 May 2017, CFDA released the Available Excipients for Health Food Filing and Their Usage Rules (Trial). There are 179 kinds of excipients allowed to use. Take three excipients as examples:
Excipient Name | Relevant standard | Maximum dosage for solid preparation | Maximum dosage for liquid preparation |
Arabic gum | GB 29949 | Appropriate | Appropriate |
Grape skin extract | GB 28313 | 2.5 g/kg | 2.5 g/kg |
Soybean oil | GB/T 1535 | Appropriate | Appropriate |
Furthermore, there are 3 additional cases that merit attention.
I. For the usage of essence, its composition shall be listed in GB 2760-2014 or Annex A of GB 30616. The dosage can be appropriate according to the production.
II. For the usage of coating premix, its composition shall be listed in GB 2760-2014 or Chinese Pharmacopoeia (2015 version). The dosage can be appropriate according to the production.
III. For the excipient used in preparing encapsulated and microencapsulated raw material, its composition shall be listed in GB 2760-2014 or the food raw materials in Health Food Raw Material Directory. The dosage can be appropriate according to the production.
Comparing with the previous drafted excipient list, there is no big difference in the formal version. The added available excipients are very little, a great many of general food materials, such as coconut oil, are still not included. The limited Excipients List will carry significant limitations to the health food filing products.
5. Main Production Processes
Currently, there are only 5 nutrition supplement forms listed the main production processes, namely, Tablet, Hard Capsule, Soft Capsule, Oral Liquid, Granule. It implies that for the moment, only above product forms are available for health food filing.
6. CIRS comments
Health food filing is much easier than health food registration, no matter for the test requirements, or the dossier requirements. With the release of the supplemental regulations, the health food filing is officially initiated now. Applicant can finally confirm the product formula and start to prepare the filing dossiers. Since the Health Food Guideline is a normative document for the filing agency and applicant, applicants are suggested to pay more attentions to the details in this document. It will help apply for the filing successfully.
Please click here for more information on health food registration and filing in China.
If you have any needs, please contact us at service@cirs-group.com.

