Breaking Tradition: Biotechnology Reshaping the Future of Food
In the wave of innovation sweeping through the food industry, synthetic biology technologies are emerging as key drivers. Whether it is enzyme conversion, whole-cell catalysis, or microbial fermentation method, these approaches are redefining how food is produced.
This article provides an in-depth analysis of these three biomanufacturing technologies—their principles, characteristics, and differences—while also exploring their current status and strategies in China’s “Three New Foods” filing system. It serves as a practical guide for innovation in the food industry.
Detailed Overview of the Three Synthetic Biology Technologies
1. Enzyme Conversion
Enzyme conversion employs enzymes extracted from microbial cells as biocatalysts to efficiently accelerate specific chemical reactions under mild conditions, catalyzing the transformation of substrates into target products.
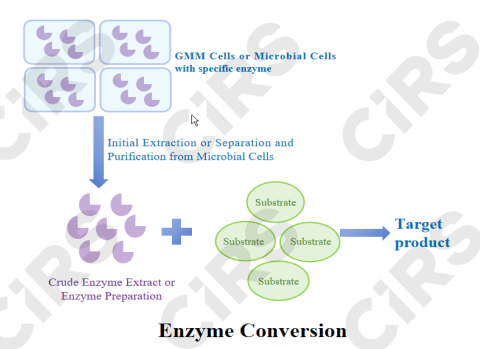
Core Principle: Enzymes, functioning as protein catalysts outside living cells, recognize substrate molecules with high specificity. By lowering the activation energy of reactions, they accelerate the transformation process without being consumed themselves.
Process Characteristics: The reaction system is relatively simple, typically comprising substrate, crude enzyme extract or enzyme preparation, and buffer system. Key parameters such as temperature and pH are controlled in the bioreactor.
Main Advantages: Mild reaction conditions (ambient temperature and pressure), high specificity (Lower byproduct levels), high catalytic efficiency, and eco-friendly.
Application Examples (successfully filed as new food raw materials or food additives in China):
Sweetener: Steviol glycosides (via enzyme conversion; Escherichia coli BL21(DE3) as chassis)
New food ingredient (sugar substitute): D-allulose (via enzyme conversion; Bacillus subtilis as chassis)
2. Whole-Cell Catalysis
Whole-cell catalysis utilizes intact microbial cells as biocatalysts, harnessing their internal enzyme systems to perform biotransformations and generate desired products.
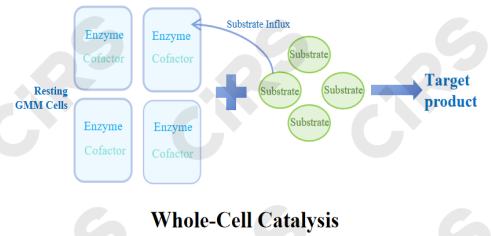
Core Principle: Using whole microbial cells (usually stay in non-growing or resting states) act as catalysts, employing their native multi-enzyme systems to perform single-step or multi-step biotransformations.
Process Characteristics: Cells with catalytic enzyme activity are harvested through fermentation and often pretreated to improve membrane permeability. Without cell growth or proliferation, substrates Influx the cells and undergo enzymatic reactions to produce the target product.
Main Advantages: No need for enzyme isolation and purification, ability to use multi-enzyme systems, cofactor regeneration, and relatively high stability.
3. Microbial Fermentation
Microbial Fermentation leverages the complete metabolic networks of living microorganisms (such as bacteria and yeasts), converting substrates into target products during growth and reproduction.
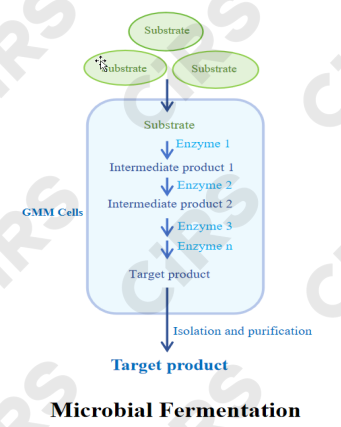
Core Principle: By providing suitable nutrients and environmental conditions, microbial cells transform substrates (such as sugars, glycerol, starch, and other carbon sources) into target products through enzymatic systems and metabolic pathways
Process Characteristics: The process involves two phases—cell growth and product synthesis (sometimes occurring simultaneously). Careful control of temperature, pH, dissolved oxygen, and nutrient supply is required.
Main Advantages: Technologically mature, suitable for large-scale production, capable of utilizing inexpensive raw materials, and effective for synthesizing complex products.
Application Examples (successfully filed as new food raw materials or food additives in China):
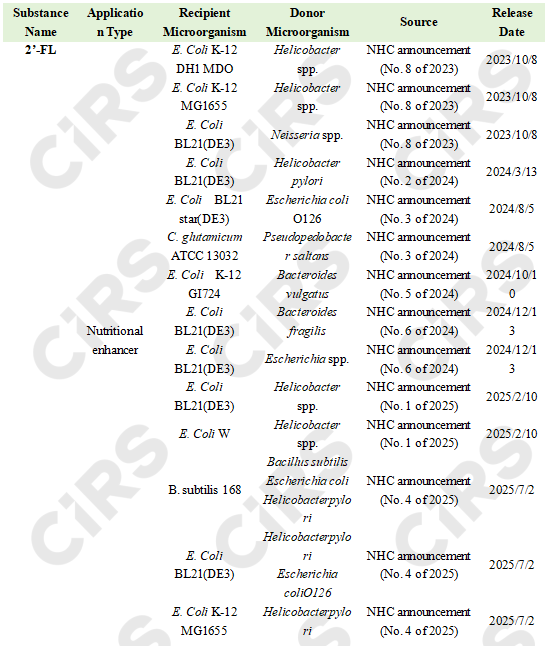
Nutritional enhancer: 2’-fucosyllactose (mainly using Escherichia coli; occasionally Bacillus subtilis and Corynebacterium glutamicum as chassis)
Nutritional enhancer: Lacto-N-neotetraose (mainly using Escherichia coli as chassis)
New food ingredient (sugar substitute): D-allulose (Escherichia coli K12 MG1655 as chassis)
Regulatory Considerations for “Three New Foods” Filings
Under China’s “Three New Foods” framework (new food ingredients, new food additives, and new food-related products), synthetic biology-based technologies must address several regulatory considerations:
Safety of Production Strains: Detailed safety evaluation of the production strains, including taxonomic identification, pathogenicity, antibiotic resistance, toxin production potential, and genetic stability.
Composition of Fermentation Products: Comprehensive analysis of the target product’s chemical structure, purity, and impurity profile.
Control of Harmful Substances: Evidence of effective control of potentially harmful metabolic by-products (e.g., organic solvents).
Process Stability: Multi-batch production data demonstrating consistency and stability of the process and product.
Current Filing Status of the Three Technologies in “Three New Foods”
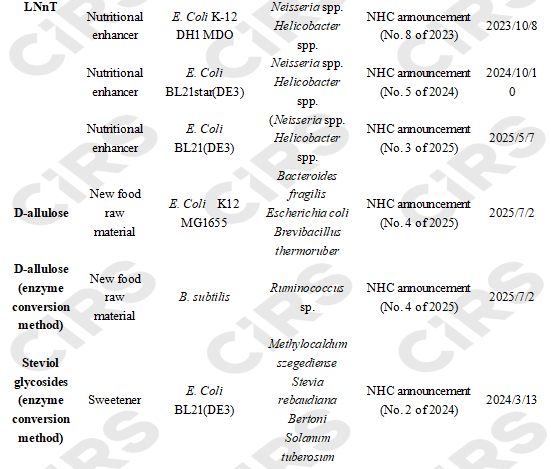
To date, 20 cases of new food ingredients and new food additives produced via microbial fermentation and enzyme conversion have been successfully filed and approved in China. The main chassis are Escherichia coli, followed by Bacillus subtilis and Corynebacterium glutamicum.
3 substances (18 products in total) produced by microbial fermentation have been successfully approved: among them, two are food nutritional enhancers 2’-fucosyllactose (14 products) and lacto-N-neotetraose (3 products); and one is a novel food ingredient: D-allulose (1 product).
2 substances (2 products in total) produced by enzyme conversion (involving genetically modified microorganisms) have been successfully approved: one novel food ingredient D-allulose (1 product), and one sweetener steviol glycosides (enzyme conversion method) (1 product).
No successful approval cases have been reported for substances produced by whole-cell catalysis.
Summary of Application Status
About CIRS Group
Established in 2012, the Food Business Division of CIRS Group has helped over 1,000 domestic and international food companies achieve one-stop compliance solutions. CIRS offers a full range of regulatory services covering novel food applications, synthetic biology-derived foods, U.S. GRAS notice, EU novel food application, health food registration, and food for special medical purposes (FSMP).
Our food services in China include but not limited to:
- China FCM Testing
- China new food raw materials registration
- China new food additive registration
- China health food (dietary supplement) registration/filing
- China health food testing service
- China new food contact substance registration
- China food for special medical purpose (FSMP) registration
- China infant formula milk powder registration
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.

