As of the end of June 2024, China's National Health Commission (NHC) has issued one announcement (No. 2 of 2024), approving a total of 23 products as “three new foods” (new food raw material, new food additive, and new food-related product). Among these, there are 12 new food additives, including those with expanded scope of use.
In this article, CIRS Group provides you with a detailed overview of the acceptance and approval status of new food additives in China for the first half of 2024.
Overview of new food additives accepted, issued for public comment, and approved in the first half of 2024
In the first half of 2024, the NHC accepted the applications for 56 new food additives. The China National Center for Food Safety Risk Assessment (CFSA) issued a total of 20 new food additives for public comment (including those with expanded scope of use). In addition, the NHC approved 12 new food additives (including those with expanded uses).
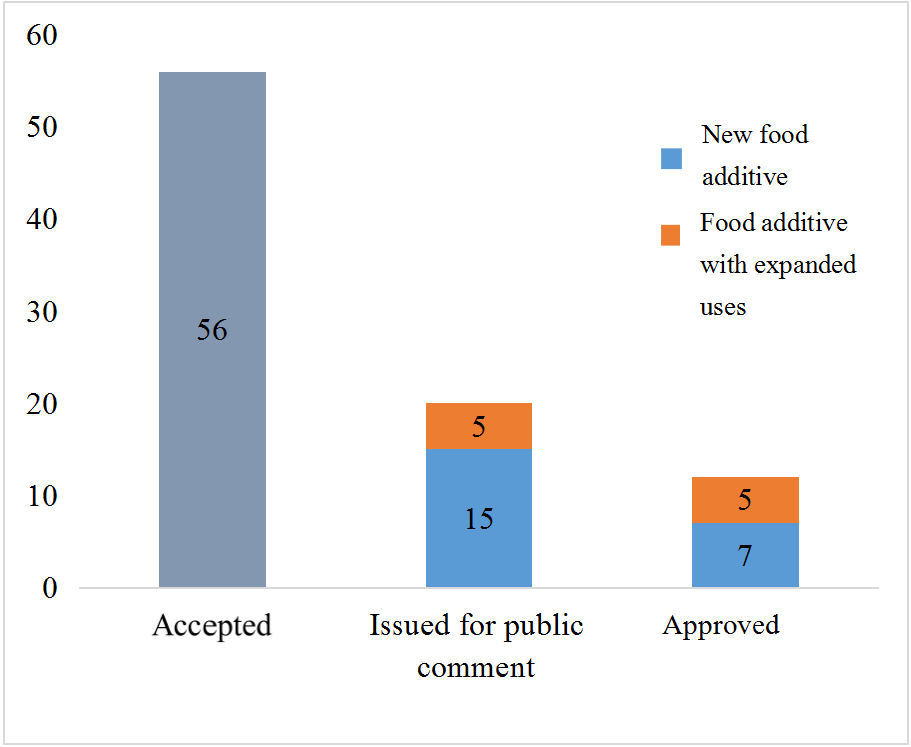
Overview of new food additives application in the first half of 2024
List of accepted new food additives in the first half of 2024 (56 types)
In the first half of 2024, the NHC accepted applications for 56 new food additives (including those with expanded scope of use). Detailed information is provided in the table below. Ten substances have been issued a non-approval decision (highlighted in blue), two have been issued a notification of review opinion (highlighted in yellow), and the remaining substances are either required to supplement materials under the extension notice or are in the public comment phase.
S.N. | Acceptance date | Acceptance No. | Substance |
|---|---|---|---|
1 | 2024-01-05 | 衛食添新申字(2024) No. 0001 | Marigold yellow |
2 | 2024-01-11 | 衛食添新申字(2024) No. 0002 | 2’-Fucosyllactose |
3 | 2024-01-12 | 衛食添新申字(2024)No. 0003 | Lacto-N-tetraose |
4 | 2024-01-16 | 衛食添新申字(2024) No. 0004 | L-alanine |
5 | 2024-01-16 | 衛食添新申字(2024) No. 0005 | 2’-Fucosyllactose |
6 | 2024-01-16 | 衛食添新申字(2024) No. 0006 | Sanzan gum |
7 | 2024-01-17 | 衛食添新申字(2024) No. 0007 | Lipase |
8 | 2024-01-18 | 衛食添新申字(2024) No. 0008 | Selenium-enriched yeast |
9 | 2024-01-18 | 衛食添新申字(2024) No. 0009 | 2’-Fucosyllactose |
10 | 2024-01-18 | 衛食添新申字(2024) No. 0010 | Chitosan |
11 | 2024-01-22 | 衛食添新申字(2024) No. 0011 | trans-Anethole Anise camphor |
12 | 2024-01-22 | 衛食添新申字(2024) No. 0012 | Citral |
13 | 2024-01-22 | 衛食添新申字(2024) No. 0013 | Hydroxycitronellal |
14 | 2024-01-23 | 衛食添新申字(2024) No. 0014 | 3’-sialyllactose sodium salt |
15 | 2024-01-24 | 衛食添新申字(2024) No. 0015 | Xylanase |
16 | 2024-01-24 | 衛食添新申字(2024) No. 0016 | D-psicose 3-epimerase |
17 | 2024-01-24 | 衛食添新申字(2024) No. 0017 | β-Alanine |
18 | 2024-01-25 | 衛食添新申字(2024) No. 0018 | Glucose oxidase |
19 | 2024-01-26 | 衛食添新申字(2024) No. 0019 | Polyglycerol polyricinoleate (polyglycerol esters of interesterified ricinoleic acid) (PGPR) |
20 | 2024-01-30 | 衛食添新申字(2024) No. 0020 | Peroxidase |
21 | 2024-02-06 | 衛食添新申字(2024) No. 0021 | Glucoamylase |
22 | 2024-02-19 | 衛食添新申字(2024) No. 0022 | Rebaudioside AM (Enzymatic conversion) |
23 | 2024-02-19 | 衛食添新申字(2024) No. 0023 | Rebaudioside M (Enzymatic conversion) |
24 | 2024-03-04 | 衛食添新申字(2024) No. 0024 | Rosemary extract |
25 | 2024-03-14 | 衛食添新申字(2024) No. 0025 | Sodium ferrous citrate |
26 | 2024-03-18 | 衛食添新申字(2024) No. 0026 | Magnesium oxide |
27 | 2024-03-18 | 衛食添新申字(2024) No. 0027 | 4-Hydroxy-2,5-dimethyl-3-(2H)-furanone |
28 | 2024-03-19 | 衛食添新申字(2024) No. 0028 | β-Alanine |
29 | 2024-03-20 | 衛食添新申字(2024) No. 0029 | Lacto-N-tetraose |
30 | 2024-03-20 | 衛食添新申字(2024) No. 0030 | Lacto-N-neotetraose |
31 | 2024-03-21 | 衛食添新申字(2024) No. 0031 | Steviol glycosides |
32 | 2024-03-21 | 衛食添新申字(2024) No. 0032 | Serine protease |
33 | 2024-03-22 | 衛食添新申字(2024) No. 0033 | Antarctic red |
34 | 2024-03-29 | 衛食添新申字(2024) No. 0034 | 6’-sialyllactose sodium salt |
35 | 2024-03-29 | 衛食添新申字(2024) No. 0035 | Glucose oxidase |
36 | 2024-03-29 | 衛食添新申字(2024) No. 0036 | Xylanase |
37 | 2024-03-29 | 衛食添新申字(2024) No. 0037 | Catalase |
38 | 2024-04-17 | 衛食添新申字(2024) No. 0038 | Recombinant human lactoferrin |
39 | 2024-04-28 | 衛食添新申字(2024) No. 0039 | Fructooligosaccharides |
40 | 2024-04-30 | 衛食添新申字(2024) No. 0040 | Steviol glycosides |
41 | 2024-05-07 | 衛食添新申字(2024) No. 0041 | Aminopeptidase |
42 | 2024-05-07 | 衛食添新申字(2024) No. 0042 | 2’-Fucosyllactose |
43 | 2024-05-10 | 衛食添新申字(2024) No. 0043 | 3,7-Dimethyl-7-hydroxyoctanal |
44 | 2024-05-10 | 衛食添新申字(2024) No. 0044 | trans-Anethole Anise camphor |
45 | 2024-05-13 | 衛食添新申字(2024) No. 0045 | Sodium cyclamate |
46 | 2024-05-14 | 衛食添新申字(2024) No. 0046 | 3-Fucosyllactose |
47 | 2024-05-17 | 衛食添新申字(2024) No. 0047 | 3’-sialyllactose sodium salt |
48 | 2024-05-20 | 衛食添新申字(2024) No. 0048 | Transglutaminase |
49 | 2024-05-20 | 衛食添新申字(2024) No. 0049 | 2’-Fucosyllactose |
50 | 2024-05-21 | 衛食添新申字(2024) No. 0050 | Lacto-N-tetraose |
51 | 2024-05-21 | 衛食添新申字(2024) No. 0051 | Lipase |
52 | 2024-05-21 | 衛食添新申字(2024) No. 0052 | Beta-glucosidase |
53 | 2024-05-24 | 衛食添新申字(2024) No. 0053 | Curdlan |
54 | 2024-06-14 | 衛食添新申字(2024) No. 0054 | Curdlan |
55 | 2024-06-18 | 衛食添新申字(2024) No. 0055 | Sucralose |
56 | 2024-06-24 | 衛食添新申字(2024) No. 0056 | Guepinia spathularia(Schw.) Fr. glycolipid |
List of new food additives that passed the technical review and were issued for public comment in the first half of 2024 (20 types)
In the first half of 2024, China’s CFSA issued 20 new food additives for public comment. These include seven new food nutrition enhancers, two new food additives, four new food enzymes, one new food processing aid, one new food flavoring, three food additives with expanded scope of use, and two nutrition enhancers with expanded scope of use. Detailed information is as follows.
List of new food nutrition enhancers issued for public comment (seven types))
S.N. | Nutrition enhancer | Scope of use | Maximum level | Production strain information | Approval status (As of the end of June 2024) |
1-3 | 2’-fucosyllactose | 01.03.02 Modified milk powder (for children only); 13.01.01 Infant formula; 13.01.02 Older infants and young children’s food; 13.01.03 Infant formula for special medical purpose | 0.7-2.4 g/L (count on a pure basis and ready-to-eat state, for powdery products, the use level should be increased by times of brewing); When mixed with lacto-N-neotetraose (LNnT), galacto-oligosaccharide (GOS), fructooligosaccharides (FOS), polyfructose, and raffinose, the total amount shall not exceed 64.5 g/kg. | Source: Escherichia coli BL21(DE3); Donor: Escherichia coli O126a | Issued for public comment on March 13, 2024. |
Source: Escherichia coli K-12 GI724; Donor: Bacteroides vulgatusa | Issued for public comment on May 10, 2024. | ||||
Source: K-12 MG1655 Escherichia coli K-12 MG1655; Donor: Helicobacter spp.a | |||||
Source: Escherichia coli BL21(DE3); Donor: Bacteroides fragilisa | Issued for public comment on June 24, 2024. | ||||
Source: Escherichia coli BL21(DE3); Donor: Escherichia spp.a | |||||
a is the donor for α-1,2-fucosyltransferase | |||||
4 | 3’-sialyllactose sodium salt, 3’-SL | 13.01.01 Infant formula; 13.01.02 Older infants and young children’s food; 13.01.03 Infant formula for special medical purpose | 0.11~0.24 g/L (count as 3’-SL and the ready-to-eat state, for powdery products, the use level should be increased by times of brewing); When mixed with 2’-FL, LNnT, 6’-sialyllactose sodium salt (6’-SL),GOS, FOS, polyfructose, and raffinose, the total amount shall not exceed 64.5 g/kg. | Source: Escherichia coli W NEO3; Donor: Saccharomyces cerevisiaea, Synechocystis spb; Rhodobacter capsulatusc; Pasteurellamultocidad; Neisseria lactamicae | 1) Accepted on September 21, 2023. Acceptance No.: 衛食添新申字(2023) No.0062; 2) Issued for public comment on March 13, 2024. |
a is the donor for N-acetylglucosamine 6-phosphate N-acetyltransferase b is the donor for N-acetylglucosamine-2-epimerase c is the donor for N-acetylserotonin synthase d is the donor for Adenosine 5’-monophosphate-N-acetylneuraminate synthase e is the donor for Alpha-2,3-sialyltransferase | |||||
5 | 6’-sialyllactose sodium salt, 6’-SL | 13.01.01 Infant formula; 13.01.02 Older infants and young children’s food; 13.01.03 Infant formula for special medical purpose | 0.14~0.40 g/L (count as 6’-SL and the ready-to-eat state, for powdery products, the use level should be increased by times of brewing); When mixed with 2’-FL, LNnT, 3’-SL, GOS, FOS, polyfructose, and raffinose, the total amount shall not exceed 64.5 g/kg. | Source: W NEO6 Escherichia coli W NEO6; Donor: Saccharomyces cerevisiaea, Synechocystis sp.b; Pasteurellamultocidac; Photobacterium damselaed; Rhodobacter capsulatuse | 1) Accepted on May 4, 2023. Acceptance No.: 衛食添新申字(2023) No.0033; 2) Issued for public comment on March 13, 2024. |
a is the donor for N-acetylglucosamine 6-phosphate N-acetyltransferase b is the donor for N-acetylglucosamine-2-epimerase c is the donor for Adenosine 5’-monophosphate-N-acetylneuraminate synthase d is the donor for Alpha-2,6-sialyltransferase e is the donor for N-acetylneuraminate synthase | |||||
6 | Lacto-N-neotetraose, LNnT | 01.03.02 Modified milk powder (for children only); 13.01.01 Infant formula; 13.01.02 Older infants and young children’s food; 13.01.03 Infant formula for special medical purpose | 0.2-0.6 g/L (count on a pure basis and ready-to-eat state, for powdery products, the use level should be increased by times of brewing); When mixed with 2’-FL, GOS, FOS, polyfructose, and raffinose, the total amount shall not exceed 64.5 g/kg.
| Source: Escherichia coli BL21 star (DE3); Donor: Neisseria spp. a and Helicobacter spp.b | 1) Accepted on March 20, 2024. Acceptance No.: 衛食添新申字(2024) No.0030; 2) Issued for public comment on May 10, 2024. |
a is the donor for β-1,3-N-acetylglucosaminyltransferase b is the donor for β-1,4-galactosyltransferase | |||||
7 | Lacto-N-tetraose, LNT | 01.03.02 Modified milk powder (for children only); 13.01.01 Infant formula; 13.01.02 Older infants and young children’s foods; 13.01.03 Infant formula for special medical purpose | 0.23-1.82 g/L (count on a pure basis and ready-to-eat state, for powdery products, the use level should be increased by times of brewing); When mixed with 2’-FL, LNnt, GOS, FOS, polyfructose, and raffinose, the total amount shall not exceed 64.5 g/kg. | Source: Escherichia coli BL21 star (DE3); Donor: Neisseria spp.a, Salmonella spp.b | Issued for public comment on June 24, 2024. |
a is the donor for β-1,3-N-acetylglucosamine aminotransferase b is the donor for β-1,3-galactosyltransferase | |||||
List of new food additives issued for public comment (two types))
S.N. | Food additive | Function | Category number | Food name/ category | Maximum level (g/kg) | Approval status (As of the end of June 2024) |
1 | Hydroxytyrosol | Antioxidant | 02.01.01 | Plant oils and fats | 0.05 | 1) Accepted on September 15, 2023. Acceptance No.: 衛食添新申字(2023) No.0054; 2) Issued for public comment on March 13, 2024. |
2 | Steviol glycosides from fermentation | Sweetener | The scope of use and use level of steviol glycosides from fermentation shall comply with the provisions of GB2760-2024 National Food Safety Standard for the Use of Food Additives. It can be used alone or in combination with steviol glycosides and enzymatically produced steviol glycosides, calculated as steviol equivalents. | Issued for public comment on June 24, 2024. | ||
Information on the production strain of steviol glycosides from fermentation is as follows: Source: Escherichia coli BL21(DE3); Donor: Taxuscanadensisa, Stevia rebaudianab, Arabidopsis thalianac, and Oryza sativad a is the donor for geranylgeranyl pyrophosphate synthase b is the donor for pyrophosphoric acid synthase, kaurenoic acid synthase, kaurenoic acid oxidase, and cytochrome P450 oxidoreductase and glycosyltransferase c is the donor for cytochrome monooxygenase d is the donor for glycosyltransferase | ||||||
List of new food enzymes issued for public comment (four types))
S.N. | Enzyme | Source | Donor | Approval status (As of the end of June 2024) |
|---|---|---|---|---|
1 | D-psicose 3-epimerase | Bacillus subtilis | Ruminococcus sp. 5_1_39B_FAA | 1) Accepted on January 24, 2024. Acceptance No.: 衛食添新申字(2024) No. 0016; 2) Issued for public comment on May 10, 2024. |
2 | Glucoamylase | Aspergillus niger | Penicillium oxalicum | 1) Accepted on November 3, 2023. Acceptance No.: 衛食添新申字(2023) No. 0066; 2) Issued for public comment on June 24, 2024. |
3 | Serine protease | Fusarium venenatum | Fusarium oxysporum | 1) Accepted on November 3, 2023. Acceptance No.: 衛食添新申字(2023) No. 0067; 2) Issued for public comment on June 24, 2024. |
4 | Lipase | Komagataella phaffi | Aspergillus oryzae | 1) Issued for public comment on June 24, 2024. |
List of new food processing aid issued for public comment (one type)
S.N. | Processing aid | Function | Scope of use | Approval status (As of the end of June 2024) |
1 | Methylene chloride | Extracting solvent | Tea decaffeination process (residue ≤ 2 mg/kg) | 1) Accepted on November 11, 2022. Acceptance No.: 衛食添新申字(2022) No. 0084; 2) Issued for public comment on March 13, 2024. |
List of new food flavoring issued for public comment (one type)
S.N. | Food flavoring | Function | Specification | Approval status (As of the end of June 2024) |
1 | 4-Hydroxy-2,5-dimethyl-3-(2H)-furanone | Food flavoring | The specifications apply to 4-Hydroxy-2,5-dimethyl-3-(2H)-furanone, a food additive produced from rhamnose. Other aspects shall comply with the provisions of GB28365 National Food Safety Standard – Food Additive - 4-Hydroxy-2,5-dimethyl-3-(2H)-furanone. | 1) Accepted on March 18, 2024. Acceptance No.: 衛食添新申字(2024) No.0027; 2) Issued for public comment on June 24, 2024. |
List of food nutrition enhancers with an expanded scope of use issued for public comment (two types)
S.N. | Nutrition enhancer | Category number | Food name/category | Maximum level | Approval status (As of the end of June 2024) |
1 | (6S)-5-methyltetrahydrofolic acid, glucosamine salt | 13.05 | Foods for special dietary purposes excluding 13.01-13.04 (nutrition supplements for pregnant and breastfeeding women only) | It shall comply with the provisions regarding folic acid specified in GB 31601 National Food Safety Standard - Nutrition Supplements for Pregnant and breastfeeding Women | 1) Accepted on September 6, 2023. Acceptance No.: 衛食添新申字(2023) No.0051; 2) Issued for public comment on March 13, 2024. |
2 | Sodium ferrous citrate | As a compound source of iron, its use level and scope of use shall comply with the provisions regarding iron in GB14880 National Food Safety Standard – Food Nutrition Enhancer. | 1) Accepted on March 14, 2024. Acceptance No.: 衛食添新申字(2024) No. 0025; 2) Issued for public comment on May 10, 2024. | ||
List of food additives with an expanded scope of use issued for public comment (three types)
S.N. | Food additive | Function | Category number | Food name/category | Maximum level (g/kg) | Note | Approval status (As of the end of June 2024) |
|---|---|---|---|---|---|---|---|
1 | Polyglycerol polyricinoleate (polyglycerol esters of interesterified ricinoleic acid) (PGPR) | Emulsifier | 01.05.03 | Formulated cream | 10.0 | — | 1) Accepted on January 26, 2024. Acceptance No.: 衛食添新申字(2024) No. 0019; 2) Issued for public comment on March 13, 2024. |
2 | Sodium cyclamate | Sweetener | 01.02.02 | Flavored fermented milk | 0.65 | Count as cyclamic acid | 1) Accepted on May 13, 2024. Acceptance No.: 衛食添新申字(2024) No. 0045; 2) Issued for public comment on June 24, 2024. |
16.07 | Others (konjac gel products only) | 1.0 | |||||
3 | Steviol glycosides | Sweetener | 01.03.02 | Formulated milk powder and formulated cream powder | 0.3 | Count as steviol equivalents | 1) Accepted on April 30, 2024. Acceptance No.: 衛食添新申字(2024) No. 0040; 2) Issued for public comment on June 24, 2024. |
01.06.04 | Cheese and processed cheese | 0.4 | |||||
04.03.02.03 | Pickled edible fungi and seaweeds | 0.23 | |||||
06.07 | Instant rice flour products | 0.4 |
List of approved new food additives in the first half of 2024 (twelve types)
In the first half of 2024, China’s NHC approved 12 new food additives. These include two new food nutrition enhancers, two new food additives, three new food enzymes, and five food additives with expanded scopes and use. Details are as follows:
Approved new food nutrition enhancers (two types)
S.N. | Nutrition enhancer | Category number | Food name/category | Maximum level | Information on acceptance, public consultation, and approval (As of the end of June 2024) |
1 | 2’-fucosyllactose | 01.03.02 | Modified milk powder (for children only) | 0.7-2.4 g/L (count on a pure basis and ready-to-eat state, for powdery products, the use level should be increased by times of brewing); When mixed with LNnT, GOS, FOS, polyfructose, and raffinose, the total amount shall not exceed 64.5 g/kg. |
|
13.01.01 | Infant formula | ||||
13.01.02 | Older infants and young children’s food | ||||
13.01.03 | Infant formula for special medical purposes | ||||
Information on the production strain of 2’-fucosyllactose: Source: Escherichia coli BL21(DE3); Donor: Helicobacter pyloria a is the donor for α-1,2-fucosyltransferase | |||||
2 | d-ribose | 13.05 | Other special dietary foods except 13.01-13.04 (only for sports nutrition foods) | 1-2 g/day | 1) Accepted on July 12, 2021. Acceptance No.: 衛食添新申字(2021) No. 0034; 2) Issued for public comment on October 21, 2021; 3) Officially approved on March 13, 2024, according to Announcement No. 2 of 2024. |
Approved new food additives (two types)
S.N. | Food additive | Function | Category number | Food name/category | Maximum level (g/L) | Note | Information on acceptance, public consultation, and approval (As of the end of June 2024) |
1 | Mixed tocotrienol tocopherol concentrate | Antioxidant | 02.01.01 | Plant fats and oils | 0.2 | Count as the total amount of tocopherol and tocotrienol | 1) Accepted on June 21, 2019. Acceptance No.: 衛食添新申字(2019) No. 0037; 2) Issued for public comment on June 26, 2023; 3) Officially approved on March 13, 2024, according to Announcement No. 2 of 2024. |
2 | Enzymatically produced steviol glycosides | Sweetener | 01.01.03 | Modified milk | 0.18 | It can be used alone or in combination with steviol glycosides. Count as steviol equivalents. | 1) Accepted on May 10, 2023. Acceptance No.: 衛食添新申字(2023) No. 0034. Acceptance name: Rebaudioside M; 2) Issued for public comment on June 26, 2023; 3) Officially approved on March 13, 2024, according to Announcement No. 2 of 2024. |
01.02.02 | Flavored fermented milk | 0.2 | |||||
03.01 | Ice cream and ice milk | 0.5 | |||||
05.02.01 | Gum-based candy | 3.5 | |||||
14.0 | Beverages (excluding 14.01 packaged drinking water, 14.02.01 fruit and vegetable juice (pulp), and 14.02.02 concentrated fruit and vegetable juice (pulp)) | 0.2 | It can be used alone or in combination with steviol glycosides. Count as steviol equivalents. Increase the usage of solid drinks according to the dilution ratio. | ||||
Information on the production strain of enzymatically produced steviol glycosides: Source: Escherichia coli BL21 (DE3); Donor: Methylocaldum szegediensea, Stevia rebaudiana Bertonib, and Solanum tuberosumc a is the donor for sucrose synthase b is the donor for β-1,3-galactosyltransferase c is the donor for β-1,2-glucosyltransferase | |||||||
Approved new food enzymes (three types)
S.N. | Food enzyme | Source | Donor | Information on acceptance, public consultation, and approval (As of the end of June 2024) |
|---|---|---|---|---|
1 | D-psicose 3-epimerase | Bacillus subtilis | Clostridium scindens ATCC35704 |
|
2 | Cyclomaltodextin glucanotransferase | Anoxybacillus caldiproteolyticus | — |
|
3 | Cellulase | Penicillium oxalicum | — |
|
Approved food additives with expanded scope and use (five types)
S.N. | Food additive | Function | Category number | Food name/category | Maximum level (g/kg) | Note | Information on acceptance, public consultation, and approval (As of the end of June 2024) |
1 | Propylene glycol alginate | Thickening agent | 06.05.02.01 | Bean vermicelli, bean noodles | 1.5 | — |
|
06.05.02.04 | Round rice ball | — | |||||
2 | Polyoxyethylene (20) sorbitan monooleat | Emulsifier | 16.03 | Collagen casing | 0.5 | — |
|
3 | Ascorbyl palmitate (enzymatic) | Antioxidant | 01.03.02 | Milk powder (including sweetened milk powder) and cream powder and the formulated products | 0.2 | Count as the ascorbic acid in fats |
|
07.01 | Bread | 0.2 | — | ||||
14.05.01 | Tea drinks | 0.2 | Count as the state of ready-to-drink, increase the usage of solid drinks according to the dilution ratio | ||||
4 | Rosemary extract | Antioxidant | 04.05.02 | Processed nuts and seeds | 0.3 | — |
|
5 | Sucralose | Sweetener | 04.05.02.01.01 | Shelled and cooked processed nuts and seeds | 4.0 | — |
|
04.05.02.01.02 | Unshelled and cooked processed nuts and seeds | 2.0 |
Conclusion
Compared to the same period last year, the number of new food additive applications accepted in the first half of 2024 has increased, and the official review process has significantly accelerated. In the future, companies should be well-prepared in terms of product safety and the technical necessity of their use to fully meet the review standards.
Since the official approval of human milk oligosaccharides (HMOs) in China last October, there have been ongoing updates on the acceptance and approval status of popular HMO substances such as 3-FL, 2’-FL, LNT, 3’-SL, 6’-SL, and LNnT. In just the first half of 2024, one 2’-FL was approved again, and five products from different production strains were opened for public comment. LNT, 3’-SL, and 6’-SL also passed the technical review and were released for public comment. It is believed that HMOs will continue to be a focal point in regulatory applications and reviews in the coming years.
Additionally, high-quality sweeteners have garnered significant market attention. The successful approval of enzymatically produced steviol glycosides in China is highly significant for the development of the sugar substitute industry. At the same time, steviol glycosides from fermentation are also in the public consultation phase, with high-quality monomers such as Rebaudioside M and AM currently under review.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
(Note: There may be omissions and inaccuracies in the data. The data in this article is for reference only. Please refer to the official information released by government departments. CIRS Group welcomes any corrections.)

